Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial
- PMID: 22615543
- PMCID: PMC3352861
- DOI: 10.1371/journal.pmed.1001217
Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial
Abstract
Background: Few data have described long-term outcomes for infants born to HIV-infected African women taking antiretroviral therapy (ART) in pregnancy. This is particularly true for World Health Organization (WHO)-recommended tenofovir-containing first-line regimens, which are increasingly used and known to cause renal and bone toxicities; concerns have been raised about potential toxicity in babies due to in utero tenofovir exposure.
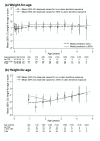
Methods and findings: Pregnancy outcome and maternal/infant ART were collected in Ugandan/Zimbabwean HIV-infected women initiating ART during The Development of AntiRetroviral Therapy in Africa (DART) trial, which compared routine laboratory monitoring (CD4; toxicity) versus clinically driven monitoring. Women were followed 15 January 2003 to 28 September 2009. Infant feeding, clinical status, and biochemistry/haematology results were collected in a separate infant study. Effect of in utero ART exposure on infant growth was analysed using random effects models. 382 pregnancies occurred in 302/1,867 (16%) women (4.4/100 woman-years [95% CI 4.0-4.9]). 226/390 (58%) outcomes were live-births, 27 (7%) stillbirths (≥22 wk), and 137 (35%) terminations/miscarriages (<22 wk). Of 226 live-births, seven (3%) infants died <2 wk from perinatal causes and there were seven (3%) congenital abnormalities, with no effect of in utero tenofovir exposure (p>0.4). Of 219 surviving infants, 182 (83%) enrolled in the follow-up study; median (interquartile range [IQR]) age at last visit was 25 (12-38) months. From mothers' ART, 62/9/111 infants had no/20%-89%/≥90% in utero tenofovir exposure; most were also zidovudine/lamivudine exposed. All 172 infants tested were HIV-negative (ten untested). Only 73/182(40%) infants were breast-fed for median 94 (IQR 75-212) days. Overall, 14 infants died at median (IQR) age 9 (3-23) months, giving 5% 12-month mortality; six of 14 were HIV-uninfected; eight untested infants died of respiratory infection (three), sepsis (two), burns (one), measles (one), unknown (one). During follow-up, no bone fractures were reported to have occurred; 12/368 creatinines and seven out of 305 phosphates were grade one (16) or two (three) in 14 children with no effect of in utero tenofovir (p>0.1). There was no evidence that in utero tenofovir affected growth after 2 years (p = 0.38). Attained height- and weight for age were similar to general (HIV-uninfected) Ugandan populations. Study limitations included relatively small size and lack of randomisation to maternal ART regimens.
Conclusions: Overall 1-year 5% infant mortality was similar to the 2%-4% post-neonatal mortality observed in this region. No increase in congenital, renal, or growth abnormalities was observed with in utero tenofovir exposure. Although some infants died untested, absence of recorded HIV infection with combination ART in pregnancy is encouraging. Detailed safety of tenofovir for pre-exposure prophylaxis will need confirmation from longer term follow-up of larger numbers of exposed children.
Trial registration: www.controlled-trials.com ISRCTN13968779
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–180. - PubMed
-
- World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 2010 revision. Geneva: WHO; 2010. - PubMed
-
- World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2010. 2010. Available: http://www.who.int/hiv/pub/2010progressreport/report/en/index.html. Accessed 18 April 2010.
-
- World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2011. 2011. Available: http://www.who.int/hiv/pub/progress_report2011/en/index.html. Accessed 10 December 2011.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

