Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis
- PMID: 22615570
- PMCID: PMC3355090
- DOI: 10.1371/journal.ppat.1002712
Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis
Abstract
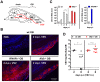
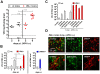
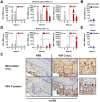
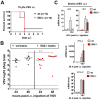
Interferon protects mice from vesicular stomatitis virus (VSV) infection and pathogenesis; however, it is not known which of the numerous interferon-stimulated genes (ISG) mediate the antiviral effect. A prominent family of ISGs is the interferon-induced with tetratricopeptide repeats (Ifit) genes comprising three members in mice, Ifit1/ISG56, Ifit2/ISG54 and Ifit3/ISG49. Intranasal infection with a low dose of VSV is not lethal to wild-type mice and all three Ifit genes are induced in the central nervous system of the infected mice. We tested their potential contributions to the observed protection of wild-type mice from VSV pathogenesis, by taking advantage of the newly generated knockout mice lacking either Ifit2 or Ifit1. We observed that in Ifit2 knockout (Ifit2(-/-)) mice, intranasal VSV infection was uniformly lethal and death was preceded by neurological signs, such as ataxia and hind limb paralysis. In contrast, wild-type and Ifit1(-/-) mice were highly protected and survived without developing such disease. However, when VSV was injected intracranially, virus replication and survival were not significantly different between wild-type and Ifit2(-/-) mice. When administered intranasally, VSV entered the central nervous system through the olfactory bulbs, where it replicated equivalently in wild-type and Ifit2(-/-) mice and induced interferon-β. However, as the infection spread to other regions of the brain, VSV titers rose several hundred folds higher in Ifit2(-/-) mice as compared to wild-type mice. This was not caused by a broadened cell tropism in the brains of Ifit2(-/-) mice, where VSV still replicated selectively in neurons. Surprisingly, this advantage for VSV replication in the brains of Ifit2(-/-) mice was not observed in other organs, such as lung and liver. Pathogenesis by another neurotropic RNA virus, encephalomyocarditis virus, was not enhanced in the brains of Ifit2(-/-) mice. Our study provides a clear demonstration of tissue-, virus- and ISG-specific antiviral action of interferon.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Interferon-induced protein Ifit2 protects mice from infection of the peripheral nervous system by vesicular stomatitis virus.J Virol. 2014 Sep;88(18):10303-11. doi: 10.1128/JVI.01341-14. Epub 2014 Jul 2. J Virol. 2014. PMID: 24991014 Free PMC article.
-
The interferon-induced protein, IFIT2, requires RNA-binding activity and neuronal expression to protect mice from intranasal vesicular stomatitis virus infection.mBio. 2024 Jul 17;15(7):e0056824. doi: 10.1128/mbio.00568-24. Epub 2024 Jun 18. mBio. 2024. PMID: 38888342 Free PMC article.
-
Sendai virus pathogenesis in mice is prevented by Ifit2 and exacerbated by interferon.J Virol. 2014 Dec;88(23):13593-601. doi: 10.1128/JVI.02201-14. Epub 2014 Sep 17. J Virol. 2014. PMID: 25231314 Free PMC article.
-
A death-promoting role for ISG54/IFIT2.J Interferon Cytokine Res. 2013 Apr;33(4):199-205. doi: 10.1089/jir.2012.0159. J Interferon Cytokine Res. 2013. PMID: 23570386 Free PMC article. Review.
-
The ISG56/IFIT1 gene family.J Interferon Cytokine Res. 2011 Jan;31(1):71-8. doi: 10.1089/jir.2010.0101. Epub 2010 Oct 15. J Interferon Cytokine Res. 2011. PMID: 20950130 Free PMC article. Review.
Cited by
-
IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon.PLoS Pathog. 2015 Apr 30;11(4):e1004863. doi: 10.1371/journal.ppat.1004863. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25927359 Free PMC article.
-
Learning from the messengers: innate sensing of viruses and cytokine regulation of immunity - clues for treatments and vaccines.Viruses. 2013 Jan 31;5(2):470-527. doi: 10.3390/v5020470. Viruses. 2013. PMID: 23435233 Free PMC article. Review.
-
Peli1 negatively regulates type I interferon induction and antiviral immunity in the CNS.Cell Biosci. 2015 Jun 24;5:34. doi: 10.1186/s13578-015-0024-z. eCollection 2015. Cell Biosci. 2015. PMID: 26131354 Free PMC article.
-
Homeostatic interferon expression in neurons is sufficient for early control of viral infection.J Neuroimmunol. 2015 Feb 15;279:11-9. doi: 10.1016/j.jneuroim.2014.12.012. Epub 2014 Dec 16. J Neuroimmunol. 2015. PMID: 25669994 Free PMC article.
-
Adar-mediated A-to-I editing is required for embryonic patterning and innate immune response regulation in zebrafish.Nat Commun. 2022 Sep 20;13(1):5520. doi: 10.1038/s41467-022-33260-6. Nat Commun. 2022. PMID: 36127363 Free PMC article.
References
-
- Detje CN, Meyer T, Schmidt H, Kreuz D, Rose JK, et al. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J Immunol. 2009;182:2297–2304. - PubMed
-
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. - PubMed
-
- Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, et al. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci U S A. 1995;92:11284–11288. - PMC - PubMed
-
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. - PubMed
-
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

