Gammaherpesvirus latency accentuates EAE pathogenesis: relevance to Epstein-Barr virus and multiple sclerosis
- PMID: 22615572
- PMCID: PMC3355105
- DOI: 10.1371/journal.ppat.1002715
Gammaherpesvirus latency accentuates EAE pathogenesis: relevance to Epstein-Barr virus and multiple sclerosis
Abstract
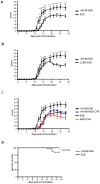
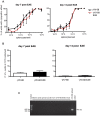
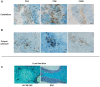
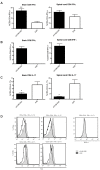
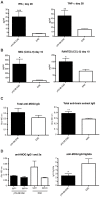
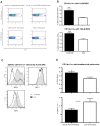
Epstein-Barr virus (EBV) has been identified as a putative environmental trigger of multiple sclerosis (MS), yet EBV's role in MS remains elusive. We utilized murine gamma herpesvirus 68 (γHV-68), the murine homolog to EBV, to examine how infection by a virus like EBV could enhance CNS autoimmunity. Mice latently infected with γHV-68 developed more severe EAE including heightened paralysis and mortality. Similar to MS, γHV-68EAE mice developed lesions composed of CD4 and CD8 T cells, macrophages and loss of myelin in the brain and spinal cord. Further, T cells from the CNS of γHV-68 EAE mice were primarily Th1, producing heightened levels of IFN-γ and T-bet accompanied by IL-17 suppression, whereas a Th17 response was observed in uninfected EAE mice. Clearly, γHV-68 latency polarizes the adaptive immune response, directs a heightened CNS pathology following EAE induction reminiscent of human MS and portrays a novel mechanism by which EBV likely influences MS and other autoimmune diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Multiple Sclerosis-Like Symptoms in Mice Are Driven by Latent γHerpesvirus-68 Infected B Cells.Front Immunol. 2020 Nov 19;11:584297. doi: 10.3389/fimmu.2020.584297. eCollection 2020. Front Immunol. 2020. PMID: 33329556 Free PMC article.
-
Latent virus infection upregulates CD40 expression facilitating enhanced autoimmunity in a model of multiple sclerosis.Sci Rep. 2015 Sep 10;5:13995. doi: 10.1038/srep13995. Sci Rep. 2015. PMID: 26356194 Free PMC article.
-
Influence of Type I Interferons in Gammaherpesvirus-68 and Its Influence on EAE Enhancement.Front Immunol. 2022 Jul 7;13:858583. doi: 10.3389/fimmu.2022.858583. eCollection 2022. Front Immunol. 2022. PMID: 35874728 Free PMC article.
-
Mechanisms regulating regional localization of inflammation during CNS autoimmunity.Immunol Rev. 2012 Jul;248(1):205-15. doi: 10.1111/j.1600-065X.2012.01126.x. Immunol Rev. 2012. PMID: 22725963 Free PMC article. Review.
-
A mouse model for infectious mononucleosis.Immunol Res. 2002;25(3):201-17. doi: 10.1385/IR:25:3:201. Immunol Res. 2002. PMID: 12018460 Review.
Cited by
-
Molecular Mimicry Analyses Unveiled the Human Herpes Simplex and Poxvirus Epitopes as Possible Candidates to Incite Autoimmunity.Pathogens. 2022 Nov 16;11(11):1362. doi: 10.3390/pathogens11111362. Pathogens. 2022. PMID: 36422613 Free PMC article.
-
Herpesvirus trigger accelerates neuroinflammation in a nonhuman primate model of multiple sclerosis.Proc Natl Acad Sci U S A. 2018 Oct 30;115(44):11292-11297. doi: 10.1073/pnas.1811974115. Epub 2018 Oct 15. Proc Natl Acad Sci U S A. 2018. PMID: 30322946 Free PMC article.
-
Animal Models for Gammaherpesvirus Infections: Recent Development in the Analysis of Virus-Induced Pathogenesis.Pathogens. 2020 Feb 12;9(2):116. doi: 10.3390/pathogens9020116. Pathogens. 2020. PMID: 32059472 Free PMC article. Review.
-
Interleukin-17 in Chronic Inflammatory Neurological Diseases.Front Immunol. 2020 Jun 3;11:947. doi: 10.3389/fimmu.2020.00947. eCollection 2020. Front Immunol. 2020. PMID: 32582147 Free PMC article. Review.
-
Presence of Epstein-Barr virus DNA in cerebrospinal fluid is associated with greater HIV RNA and inflammation.AIDS. 2020 Mar 1;34(3):373-380. doi: 10.1097/QAD.0000000000002442. AIDS. 2020. PMID: 31764071 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

