Deletion of PTH rescues skeletal abnormalities and high osteopontin levels in Klotho-/- mice
- PMID: 22615584
- PMCID: PMC3355080
- DOI: 10.1371/journal.pgen.1002726
Deletion of PTH rescues skeletal abnormalities and high osteopontin levels in Klotho-/- mice
Abstract
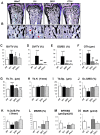
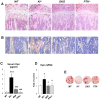
Maintenance of normal mineral ion homeostasis is crucial for many biological activities, including proper mineralization of the skeleton. Parathyroid hormone (PTH), Klotho, and FGF23 have been shown to act as key regulators of serum calcium and phosphate homeostasis through a complex feedback mechanism. The phenotypes of Fgf23(-/-) and Klotho(-/-) (Kl(-/-)) mice are very similar and include hypercalcemia, hyperphosphatemia, hypervitaminosis D, suppressed PTH levels, and severe osteomalacia/osteoidosis. We recently reported that complete ablation of PTH from Fgf23(-/-) mice ameliorated the phenotype in Fgf23(-/-)/PTH(-/-) mice by suppressing serum vitamin D and calcium levels. The severe osteomalacia in Fgf23(-/-) mice, however, persisted, suggesting that a different mechanism is responsible for this mineralization defect. In the current study, we demonstrate that deletion of PTH from Kl(-/-) (Kl(-/-)/PTH(-/-) or DKO) mice corrects the abnormal skeletal phenotype. Bone turnover markers are restored to wild-type levels; and, more importantly, the skeletal mineralization defect is completely rescued in Kl(-/-)/PTH(-/-) mice. Interestingly, the correction of the osteomalacia is accompanied by a reduction in the high levels of osteopontin (Opn) in bone and serum. Such a reduction in Opn levels could not be observed in Fgf23(-/-)/PTH(-/-) mice, and these mice showed sustained osteomalacia. This significant in vivo finding is corroborated by in vitro studies using calvarial osteoblast cultures that show normalized Opn expression and rescued mineralization in Kl(-/-)/PTH(-/-) mice. Moreover, continuous PTH infusion of Kl(-/-) mice significantly increased Opn levels and osteoid volume, and decreased trabecular bone volume. In summary, our results demonstrate for the first time that PTH directly impacts the mineralization disorders and skeletal deformities of Kl(-/-), but not of Fgf23(-/-) mice, possibly by regulating Opn expression. These are significant new perceptions into the role of PTH in skeletal and disease processes and suggest FGF23-independent interactions of PTH with Klotho.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



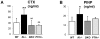

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

