An efficient procedure for purification of recombinant human β heat shock protein 90
- PMID: 22615596
- PMCID: PMC3232084
An efficient procedure for purification of recombinant human β heat shock protein 90
Abstract
Background and the purpose of the study: Heat Shock Protein 90 (Hsp90) is typically the most abundant chaperone in the eukaryotic cell cytoplasm, and its expression is essential for loading immunogenic peptides onto major histocompatibility complex molecules for presentation to T-cells. Therefore, it may act as a good candidate as an adjuvant molecule in vaccine technology.
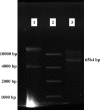
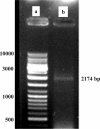
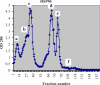
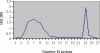
Methods: Initially the human Hsp90β gene was cloned into the heat inducible expression vector pGP1-2 and then the recombinant protein was isolated by ion exchange chromatography. After intradermal injection of confirmed purified band of protein to rabbits and isolation of the serum IgG antibody, for its affinity purification, the rabbit's purified Hsp90 specific IgG was coupled to the cyanogen bromide-activated Sepharose 4B.
Results: The recovery of the purified protein of interest by affinity chromatography was 50%.
Conclusion: This research enabled purification of human heat shock protein by a laboratory prepared column chromatography.
Keywords: CNBr activated Sepharose 4B; Head shock protein 90(Hsp90); Rabbit IgG; pGP1-2.
Figures





Similar articles
-
Development of an immunoaffinity method for purification of streptokinase.Avicenna J Med Biotechnol. 2012 Jul;4(3):142-7. Avicenna J Med Biotechnol. 2012. PMID: 23408770 Free PMC article.
-
Immunochemical studies of the non-specific interactions of cyanogen bromide-activated macroporous agarose-based immunoadsorbents.J Chromatogr. 1983 Dec 23;281:83-93. doi: 10.1016/s0021-9673(01)87868-7. J Chromatogr. 1983. PMID: 6668343
-
Evaluation by ELISA of anisakis simplex larval antigen purified by affinity chromatography.Mem Inst Oswaldo Cruz. 2002 Mar;97(2):247-52. doi: 10.1590/s0074-02762002000200018. Mem Inst Oswaldo Cruz. 2002. PMID: 12016451
-
Purification of the 90 kDa heat shock protein (hsp90) and simultaneous purification of hsp70/hsc70, hsp90 and hsp96 from mammalian tissues and cells using thiophilic interaction chromatography.Biomed Chromatogr. 2009 Nov;23(11):1208-16. doi: 10.1002/bmc.1245. Biomed Chromatogr. 2009. PMID: 19488974
-
Expressed as the sole Hsp90 of yeast, the alpha and beta isoforms of human Hsp90 differ with regard to their capacities for activation of certain client proteins, whereas only Hsp90beta generates sensitivity to the Hsp90 inhibitor radicicol.FEBS J. 2007 Sep;274(17):4453-63. doi: 10.1111/j.1742-4658.2007.05974.x. Epub 2007 Aug 6. FEBS J. 2007. PMID: 17681020
Cited by
-
Affinity Purification of Tumor Necrosis Factor-α Expressed in Raji Cells by Produced scFv Antibody Coupled CNBr-Activated Sepharose.Adv Pharm Bull. 2013;3(1):19-23. doi: 10.5681/apb.2013.004. Epub 2013 Feb 7. Adv Pharm Bull. 2013. PMID: 24312807 Free PMC article.
-
Using recombinant Chlamydia trachomatis OMP2 as antigen in diagnostic ELISA test.Iran J Microbiol. 2014 Feb;6(1):8-13. Iran J Microbiol. 2014. PMID: 25954485 Free PMC article.
References
-
- Barginear MF, Van PC, Rosen N, Modi S, Hudis CA, Budman DR. The heat shock protein 90 chaperone complex: an evolving therapeutic target. Curr Cancer Drug Tar. 2008;8:522–532. - PubMed
-
- Smith DF, Whiteseli L, Katsanis E. Molecular Chaperones: Biology and Prospects for Pharmacological Intervention. Pharmacol Rev. 1998;4:493–514. - PubMed
-
- Minami M, Nakamura M, Emori Y, Minami Y. Both the N- and C- terminal chaperone sites of Hsp90 participate in protein refolding. Eur J Biochem. 2001;268:2520–2524. - PubMed
-
- Przepiorka D, Srivastava PK. Heat Shock protein-peptide complexes as immunotherapy for human cancer. Mol Med Today. 1998;4:478–484. - PubMed
-
- Basu S, Srivastava P. Fever-like temperature induces maturation of dendritic cells through induction of Hsp90. Int Immunol. 2003;15:1053–1061. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
