Clinical efficacy and safety of polyethylene glycol 3350 versus liquid paraffin in the treatment of pediatric functional constipation
- PMID: 22615652
- PMCID: PMC3232099
Clinical efficacy and safety of polyethylene glycol 3350 versus liquid paraffin in the treatment of pediatric functional constipation
Abstract
Background and the purpose of the study: Functional constipation is prevalent in children. Recently polyethylene glycol has been introduced as an effective and safe drug to treat chronic constipation. There are only a few clinical trials on comparison of PEG and liquid paraffin in childhood constipation. The purpose of this study was to evaluate clinical efficacy and safety of PEG 3350 solution and liquid paraffin in the treatment of children with functional constipation in Sari Toba clinic during the period of 2008-2009.
Methods: Children with a history of functional constipation were subjects of this study. One hundred and sixty children of 2-12 years old with functional constipation were randomized in two PEG and paraffin treatment groups. Patients received either 1.0-1.5 g/kg/day PEG 3350 or 1.0-1.5 ml/kg/day liquid paraffin for 4 months. Clinical efficacy was evaluated by stool and encopresis frequency/week and overall treatment success rate was compared in two groups.
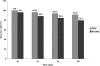
Results and major conclusion: Compared with the baseline, defecation frequency/ week increased significantly and encopresis frequency meaningfully decreased in two groups during the period of the study. Patients using PEG 3350 had more success rate (mean: 95.3%±3.7) compared with the patients in paraffin group (mean: 87.2%±7.1) (p=0.087). Administration of PEG 3350 were associated with less adverse events than liquid paraffin. In conclusion in treatment of pediatric functional constipation, regarding clinical efficacy and safety, PEG 3350 were at least as effective as liquid paraffin and but less adverse drug events.
Keywords: Defecation; Encopresis; Lactoluse; Mineral oil..
Figures
References
-
- Clinical Practice Guideline. Evaluation and Treatment of Constipation in Infants and Children: Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Journal of Pediatric Gastroenterology and Nutrition. JPGN. 2006 Sep;43:e1Ye13. - PubMed
-
- Felt B, Brown P, Coran A, Kochhar P, Opipari-Arrigan L. Functional constipation and soiling in children. University of Michigan Health System guidelines for clinical Care. 2003. Accessed online February 2, 2005, at: http://cme.med.umich.edu/pdf/guideline/peds03.pdf.
-
- Baker SS, Liptak GS, Colletti RB, Croffie JM, Di Lorenzo C, Nurko S. Constipation in infants and children: evaluation and treatment. A medical position statement of the North American Society for Pediatric Gastroenterology and nutrition. J Pediatr Gastroenterol Nutr. 1999;29:612–626. - PubMed
-
- McClung HJ, Boyne LJ, Linsheid T, Heitlinger LA, Murray RD, Fyda J, Li B U.K. Is combination therapy for encopresis nutritionally safe? Pediatr. 1993;91:591–594. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials