Selective rapid eye movement sleep deprivation affects cell size and number in kitten locus coeruleus
- PMID: 22615706
- PMCID: PMC3351802
- DOI: 10.3389/fneur.2012.00069
Selective rapid eye movement sleep deprivation affects cell size and number in kitten locus coeruleus
Abstract
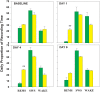
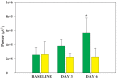
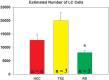
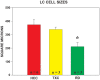
Cells in the locus coeruleus (LC) constitute the sole source of norepinephrine (NE) in the brain and change their discharge rates according to vigilance state. In addition to its well established role in vigilance, NE affects synaptic plasticity in the postnatal critical period (CP) of development. One form of CP synaptic plasticity affected by NE results from monocular occlusion, which leads to physiological and cytoarchitectural alterations in central visual areas. Selective suppression of rapid eye movement sleep (REMS) in the CP kitten enhances the central effects of monocular occlusion. The mechanisms responsible for heightened cortical plasticity following REMS deprivation (REMSD) remain undetermined. One possible mediator of an increase in plasticity is continuous NE outflow, which presumably persists during extended periods of REMSD. Tyrosine hydroxylase (TH) is the rate-limiting enzyme in the synthesis of NE and serves as a marker for NE-producing cells. We selectively suppressed REMS in kittens for 1 week during the CP. The number and size of LC cells expressing immunoreactivity to tyrosine hydroxylase (TH-ir) was assessed in age-matched REMS-deprived (RD)-, treatment-control (TXC)-, and home cage-reared (HCC) animals. Sleep amounts and slow wave activity (SWA) were also examined relative to baseline. Time spent in REMS during the study was lower in RD compared to TXC animals, and RD kittens increased SWA delta power in the latter half of the REMSD period. The estimated total number of TH-ir cells in LC was significantly lower in the RD than in the TXC kittens and numerically lower than in the HCC animals. The size of LC cells expressing TH-ir was greatest in the HCC group. HCC cells were significantly larger than TH-ir cells in the RD kittens. These data are consistent with presumed reduction in NE in forebrain areas, including visual cortex, caused by 1 week of REMSD.
Keywords: critical period; depression; fast Fourier transforms; norepinephrine; stereology.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

