Serratamolide is a hemolytic factor produced by Serratia marcescens
- PMID: 22615766
- PMCID: PMC3353980
- DOI: 10.1371/journal.pone.0036398
Serratamolide is a hemolytic factor produced by Serratia marcescens
Abstract
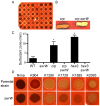
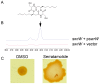
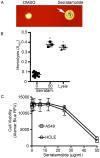
Serratia marcescens is a common contaminant of contact lens cases and lenses. Hemolytic factors of S. marcescens contribute to the virulence of this opportunistic bacterial pathogen. We took advantage of an observed hyper-hemolytic phenotype of crp mutants to investigate mechanisms of hemolysis. A genetic screen revealed that swrW is necessary for the hyper-hemolysis phenotype of crp mutants. The swrW gene is required for biosynthesis of the biosurfactant serratamolide, previously shown to be a broad-spectrum antibiotic and to contribute to swarming motility. Multicopy expression of swrW or mutation of the hexS transcription factor gene, a known inhibitor of swrW expression, led to an increase in hemolysis. Surfactant zones and expression from an swrW-transcriptional reporter were elevated in a crp mutant compared to the wild type. Purified serratamolide was hemolytic to sheep and murine red blood cells and cytotoxic to human airway and corneal limbal epithelial cells in vitro. The swrW gene was found in the majority of contact lens isolates tested. Genetic and biochemical analysis implicate the biosurfactant serratamolide as a hemolysin. This novel hemolysin may contribute to irritation and infections associated with contact lens use.
Conflict of interest statement
Figures


References
-
- Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–854. - PubMed
-
- Steppberger K, Walter S, Claros MC, Spencker FB, Kiess W, et al. Nosocomial neonatal outbreak of Serratia marcescens–analysis of pathogens by pulsed field gel electrophoresis and polymerase chain reaction. Infection. 2002;30:277–281. - PubMed
-
- Vonberg RP, Gastmeier P. Hospital-acquired infections related to contaminated substances. J Hosp Infect. 2007;65:15–23. - PubMed
-
- Holden BA, La Hood D, Grant T, Newton-Howes J, Baleriola-Lucas C, et al. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. Clao J. 1996;22:47–52. - PubMed
-
- Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, et al. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

