Phenotypic and functional changes in blood monocytes following adherence to endothelium
- PMID: 22615904
- PMCID: PMC3352879
- DOI: 10.1371/journal.pone.0037091
Phenotypic and functional changes in blood monocytes following adherence to endothelium
Abstract
Objective: Blood monocytes are known to express endothelial-like genes during co-culture with endothelium. In this study, the time-dependent change in the phenotype pattern of primary blood monocytes after adhering to endothelium is reported using a novel HLA-A2 mistyped co-culture model.
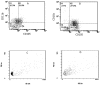
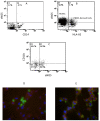
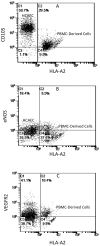
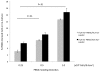
Methods and results: Freshly isolated human PBMCs were co-cultured with human umbilical vein endothelial cells or human coronary arterial endothelial cells of converse human leukocyte antigen A2 (HLA-A2) status. This allows the tracking of the PBMC-derived cells by HLA-A2 expression and assessment of their phenotype pattern over time. PBMCs that adhered to the endothelium at the start of the co-culture were predominantly CD11b+ blood monocytes. After 24 to 72 hours in co-culture, the endothelium-adherent monocytes acquired endothelial-like properties including the expression of endothelial nitric oxide synthase, CD105, CD144 and vascular endothelial growth factor receptor 2. The expression of monocyte/macrophage lineage antigens CD14, CD11b and CD36 were down regulated concomitantly. The adherent monocytes did not express CD115 after 1 day of co-culture. By day 6, the monocyte-derived cells expressed vascular cell adhesion molecule 1 in response to tumour necrosis factor alpha. Up to 10% of the PBMCs adhered to the endothelium. These monocyte-derived cells contributed up to 30% of the co-cultured cell layer and this was dose-dependent on the PBMC seeding density.
Conclusions: Human blood monocytes undergo rapid phenotype change to resemble endothelial cells after adhering to endothelium.
Conflict of interest statement
Figures




References
-
- Schubert SY, Benarroch A, Ostvang J, Edelman ER. Regulation of endothelial cell proliferation by primary monocytes. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:97–104. - PubMed
-
- Fernandez Pujol B, Lucibello FC, Gehling UM, Lindemann K, Weidner N, et al. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65:287–300. - PubMed
-
- Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, et al. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. - PubMed
-
- Bellik L, Musilli C, Vinci MC, Ledda F, Parenti A. Human mature endothelial cells modulate peripheral blood mononuclear cell differentiation toward an endothelial phenotype. Experimental cell research. 2008;314:2965–2974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

