LGR6 is a high affinity receptor of R-spondins and potentially functions as a tumor suppressor
- PMID: 22615920
- PMCID: PMC3355120
- DOI: 10.1371/journal.pone.0037137
LGR6 is a high affinity receptor of R-spondins and potentially functions as a tumor suppressor
Abstract
Background: LGR6 (leucine-rich repeat containing, G protein-coupled receptor 6) is a member of the rhodopsin-like seven transmembrane domain receptor superfamily with the highest homology to LGR4 and LGR5. LGR6 was found as one of the novel genes mutated in colon cancer through total exon sequencing and its promoter region is hypermethylated in 20-50% of colon cancer cases. In the skin, LGR6 marks a population of stem cells that can give rise to all cell lineages. Recently, we and others demonstrated that LGR4 and LGR5 function as receptors of R-spondins to potentiate Wnt/β-catenin signaling. However, the binding affinity and functional response of LGR6 to R-spondins, and the activity of colon cancer mutants of LGR6 have not been determined.
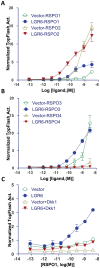
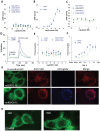
Principal findings: We found that LGR6 also binds and responds to R-spondins 1-3 with high affinity to enhance Wnt/β-catenin signaling through increased LRP6 phosphorylation. Similar to LGR4 and LGR5, LGR6 is not coupled to heterotrimeric G proteins or to β-arrestin following R-spondin stimulation. Functional and expression analysis of three somatic mutations identified in colon cancer samples indicates that one mutant fails to bind and respond to R-spondin (loss-of-function), but the other two have no significant effect on receptor function. Overexpression of wild-type LGR6 in HeLa cells leads to increased cell migration following co-treatment with R-spondin1 and Wnt3a when compared to vector control cells or cells overexpressing the loss-of-function mutant.
Conclusions: LGR6 is a high affinity receptor for R-spondins 1-3 and potentially functions as a tumor suppressor despite its positive effect on Wnt/β-catenin signaling.
Conflict of interest statement
Figures



References
-
- Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, et al. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol. 2000;14:1257–1271. - PubMed
-
- Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–1696. - PubMed
-
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. - PubMed
-
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

