Developmental hippocampal neuroplasticity in a model of nicotine replacement therapy during pregnancy and breastfeeding
- PMID: 22615944
- PMCID: PMC3352874
- DOI: 10.1371/journal.pone.0037219
Developmental hippocampal neuroplasticity in a model of nicotine replacement therapy during pregnancy and breastfeeding
Abstract
Rationale: The influence of developmental nicotine exposure on the brain represents an important health topic in light of the popularity of nicotine replacement therapy (NRT) as a smoking cessation method during pregnancy.
Objectives: In this study, we used a model of NRT during pregnancy and breastfeeding to explore the consequences of chronic developmental nicotine exposure on cerebral neuroplasticity in the offspring. We focused on two dynamic lifelong phenomena in the dentate gyrus (DG) of the hippocampus that are highly sensitive to the environment: granule cell neurogenesis and long-term potentiation (LTP).
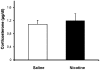
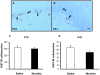
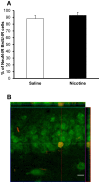
Methods: Pregnant rats were implanted with osmotic mini-pumps delivering either nicotine or saline solutions. Plasma nicotine and metabolite levels were measured in dams and offspring. Corticosterone levels, DG neurogenesis (cell proliferation, survival and differentiation) and glutamatergic electrophysiological activity were measured in pups.
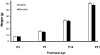
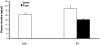
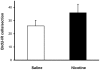
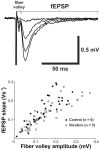
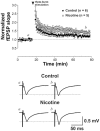
Results: Juvenile (P15) and adolescent (P41) offspring exposed to nicotine throughout prenatal and postnatal development displayed no significant alteration in DG neurogenesis compared to control offspring. However, NRT-like nicotine exposure significantly increased LTP in the DG of juvenile offspring as measured in vitro from hippocampal slices, suggesting that the mechanisms underlying nicotine-induced LTP enhancement previously described in adult rats are already functional in pups.
Conclusions: These results indicate that synaptic plasticity is disrupted in offspring breastfed by dams passively exposed to nicotine in an NRT-like fashion.
Conflict of interest statement
Figures
Similar articles
-
Differential effects of the histamine H(3) receptor agonist methimepip on dentate granule cell excitability, paired-pulse plasticity and long-term potentiation in prenatal alcohol-exposed rats.Alcohol Clin Exp Res. 2014 Jul;38(7):1902-11. doi: 10.1111/acer.12430. Epub 2014 May 12. Alcohol Clin Exp Res. 2014. PMID: 24818819 Free PMC article.
-
Impaired in vivo synaptic plasticity in dentate gyrus and spatial memory in juvenile rats induced by prenatal morphine exposure.Hippocampus. 2009 Jul;19(7):649-57. doi: 10.1002/hipo.20540. Hippocampus. 2009. PMID: 19115391
-
Aberrant neurogenesis and late onset suppression of synaptic plasticity as well as sustained neuroinflammation in the hippocampal dentate gyrus after developmental exposure to ethanol in rats.Toxicology. 2021 Oct;462:152958. doi: 10.1016/j.tox.2021.152958. Epub 2021 Sep 20. Toxicology. 2021. PMID: 34547370
-
Impaired Bidirectional Synaptic Plasticity in Juvenile Offspring Following Prenatal Ethanol Exposure.Alcohol Clin Exp Res. 2019 Oct;43(10):2153-2166. doi: 10.1111/acer.14170. Epub 2019 Aug 26. Alcohol Clin Exp Res. 2019. PMID: 31386206 Free PMC article.
-
Long-term consequences of fetal and neonatal nicotine exposure: a critical review.Toxicol Sci. 2010 Aug;116(2):364-74. doi: 10.1093/toxsci/kfq103. Epub 2010 Apr 2. Toxicol Sci. 2010. PMID: 20363831 Free PMC article. Review.
Cited by
-
In utero exposure to maternal smoking is associated with DNA methylation alterations and reduced neuronal content in the developing fetal brain.Epigenetics Chromatin. 2017 Jan 26;10:4. doi: 10.1186/s13072-017-0111-y. eCollection 2017. Epigenetics Chromatin. 2017. PMID: 28149327 Free PMC article.
-
Chronic nicotine exposure induces molecular and transcriptomic endophenotypes associated with mood and anxiety disorders in a cerebral organoid neurodevelopmental model.Front Pharmacol. 2024 Dec 23;15:1473213. doi: 10.3389/fphar.2024.1473213. eCollection 2024. Front Pharmacol. 2024. PMID: 39764466 Free PMC article.
-
Early Life Exposure to Nicotine: Postnatal Metabolic, Neurobehavioral and Respiratory Outcomes and the Development of Childhood Cancers.Toxicol Sci. 2020 Nov 1;178(1):3-15. doi: 10.1093/toxsci/kfaa127. Toxicol Sci. 2020. PMID: 32766841 Free PMC article. Review.
-
Prenatal exposure to nicotine stimulates neurogenesis of orexigenic peptide-expressing neurons in hypothalamus and amygdala.J Neurosci. 2013 Aug 21;33(34):13600-11. doi: 10.1523/JNEUROSCI.5835-12.2013. J Neurosci. 2013. PMID: 23966683 Free PMC article.
-
Performance of motor associated behavioural tests following chronic nicotine administration.Ann Neurosci. 2014 Apr;21(2):42-6. doi: 10.5214/ans.0972.7531.210203. Ann Neurosci. 2014. PMID: 25206059 Free PMC article.
References
-
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–140. - PubMed
-
- Bardy AH, Seppala T, Lillsunde P, Kataja JM, Koskela P, et al. Objectively measured tobacco exposure during pregnancy: neonatal effects and relation to maternal smoking. Br J Obstet Gynaecol. 1993;100:721–726. - PubMed
-
- Sanghavi DM. Epidemiology of sudden infant death syndrome (SIDS) for Kentucky infants born in 1990: maternal, prenatal, and perinatal risk factors. J Ky Med Assoc. 1995;93:286–290. - PubMed
-
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Hum Dev. 2003;75:21–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

