A rat model of central venous catheter to study establishment of long-term bacterial biofilm and related acute and chronic infections
- PMID: 22615964
- PMCID: PMC3353920
- DOI: 10.1371/journal.pone.0037281
A rat model of central venous catheter to study establishment of long-term bacterial biofilm and related acute and chronic infections
Abstract
Formation of resilient biofilms on medical devices colonized by pathogenic microorganisms is a major cause of health-care associated infection. While in vitro biofilm analyses led to promising anti-biofilm approaches, little is known about their translation to in vivo situations and on host contribution to the in vivo dynamics of infections on medical devices. Here we have developed an in vivo model of long-term bacterial biofilm infections in a pediatric totally implantable venous access port (TIVAP) surgically placed in adult rats. Using non-invasive and quantitative bioluminescence, we studied TIVAP contamination by clinically relevant pathogens, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Staphylococcus epidermidis, and we demonstrated that TIVAP bacterial populations display typical biofilm phenotypes. In our study, we showed that immunocompetent rats were able to control the colonization and clear the bloodstream infection except for up to 30% that suffered systemic infection and death whereas none of the immunosuppressed rats survived the infection. Besides, we mimicked some clinically relevant TIVAP associated complications such as port-pocket infection and hematogenous route of colonization. Finally, by assessing an optimized antibiotic lock therapy, we established that our in vivo model enables to assess innovative therapeutic strategies against bacterial biofilm infections.
Conflict of interest statement
Figures

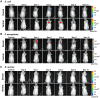
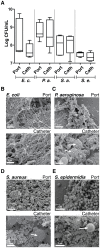
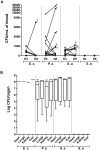

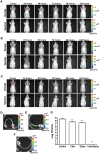
References
-
- Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cellular Microbiology. 2009;11:1034–1043. - PubMed
-
- Lynch AS, Robertson GT. Bacterial and fungal biofilm infections. Annu Rev Med. 2008;59:415–428. - PubMed
-
- Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. - PubMed
-
- Wagner C, Hänsch GM, Wentzensen A, Heppert V. Implant-associated post-traumatic osteomyelitis. Bacterial biofilms and the immune defence as protagonists of the local inflammatory process. Unfallchirurg. 2006;109:761–769. - PubMed
-
- Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

