UBR2 of the N-end rule pathway is required for chromosome stability via histone ubiquitylation in spermatocytes and somatic cells
- PMID: 22616001
- PMCID: PMC3355131
- DOI: 10.1371/journal.pone.0037414
UBR2 of the N-end rule pathway is required for chromosome stability via histone ubiquitylation in spermatocytes and somatic cells
Abstract
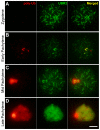
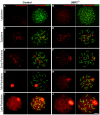
The N-end rule pathway is a proteolytic system in which its recognition components (N-recognins) recognize destabilizing N-terminal residues of short-lived proteins as an essential element of specific degrons, called N-degrons. The RING E3 ligases UBR2 and UBR1 are major N-recognins that share size (200 kDa), conserved domains and substrate specificities to N-degrons. Despite the known function of the N-end rule pathway in degradation of cytosolic proteins, the major phenotype of UBR2-deficient male mice is infertility caused by arrest of spermatocytes at meiotic prophase I. UBR2-deficient spermatocytes are impaired in transcriptional silencing of sex chromosome-linked genes and ubiquitylation of histone H2A. In this study we show that the recruitment of UBR2 to meiotic chromosomes spatiotemporally correlates to the induction of chromatin-associated ubiquitylation, which is significantly impaired in UBR2-deficient spermatocytes. UBR2 functions as a scaffold E3 that promotes HR6B/UbcH2-dependent ubiquitylation of H2A and H2B but not H3 and H4, through a mechanism distinct from typical polyubiquitylation. The E3 activity of UBR2 in histone ubiquitylation is allosterically activated by dipeptides bearing destabilizing N-terminal residues. Insufficient monoubiquitylation and polyubiquitylation on UBR2-deficient meiotic chromosomes correlate to defects in double strand break (DSB) repair and other meiotic processes, resulting in pachytene arrest at stage IV and apoptosis. Some of these functions of UBR2 are observed in somatic cells, in which UBR2 is a chromatin-binding protein involved in chromatin-associated ubiquitylation upon DNA damage. UBR2-deficient somatic cells show an array of chromosomal abnormalities, including hyperproliferation, chromosome instability, and hypersensitivity to DNA damage-inducing reagents. UBR2-deficient mice enriched in C57 background die upon birth with defects in lung expansion and neural development. Thus, UBR2, known as the recognition component of a major cellular proteolytic system, is associated with chromatin and controls chromatin dynamics and gene expression in both germ cells and somatic cells.
Conflict of interest statement
Figures









References
-
- Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. - PubMed
-
- Sriram SM, Kim BY, Kwon YT. The N-end rule pathway: emerging functions and molecular principles of substrate recognition. Nat Rev Mol Cell Biol. 2011;12:735–747. - PubMed
-
- Sriram SM, Kwon YT. The molecular principles of N-end rule recognition. Nat Strct Mol Biol. 2010;17:1164–1165. - PubMed
-
- Tasaki T, Kwon YT. The mammalian N-end rule pathway: new insights into its components and physiological roles. Trends Biochem Sci. 2007;32:520–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

