Rolling the human amnion to engineer laminated vascular tissues
- PMID: 22616610
- PMCID: PMC3483053
- DOI: 10.1089/ten.TEC.2012.0119
Rolling the human amnion to engineer laminated vascular tissues
Abstract
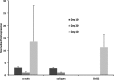
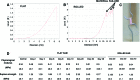
The prevalence of cardiovascular disease and the limited availability of suitable autologous transplant vessels for coronary and peripheral bypass surgeries is a significant clinical problem. A great deal of progress has been made over recent years to develop biodegradable materials with the potential to remodel and regenerate vascular tissues. However, the creation of functional biological scaffolds capable of withstanding vascular stress within a clinically relevant time frame has proved to be a challenging proposition. As an alternative approach, we report the use of a multilaminate rolling approach using the human amnion to generate a tubular construct for blood vessel regeneration. The human amniotic membrane was decellularized by agitation in 0.03% (w/v) sodium dodecyl sulfate to generate an immune compliant material. The adhesion of human umbilical vein endothelial cells (EC) and human vascular smooth muscle cells (SMC) was assessed to determine initial binding and biocompatibility (monocultures). Extended cultures were either assessed as flat membranes, or rolled to form concentric multilayered conduits. Results showed positive EC adhesion and a progressive repopulation by SMC. Functional changes in SMC gene expression and the constructs' bulk mechanical properties were concomitant with vessel remodeling as assessed over a 40-day culture period. A significant advantage with this approach is the ability to rapidly produce a cell-dense construct with an extracellular matrix similar in architecture and composition to natural vessels. The capacity to control physical parameters such as vessel diameter, wall thickness, shape, and length are critical to match vessel compliance and tailor vessel specifications to distinct anatomical locations. As such, this approach opens new avenues in a range of tissue regenerative applications that may have a much wider clinical impact.
Figures






Similar articles
-
Tuning scaffold mechanics by laminating native extracellular matrix membranes and effects on early cellular remodeling.J Biomed Mater Res A. 2014 May;102(5):1325-33. doi: 10.1002/jbm.a.34791. Epub 2013 Jun 11. J Biomed Mater Res A. 2014. PMID: 23666819 Free PMC article.
-
Porcine small diameter arterial extracellular matrix supports endothelium formation and media remodeling forming a promising vascular engineered biograft.Tissue Eng Part A. 2012 Feb;18(3-4):411-22. doi: 10.1089/ten.TEA.2011.0173. Epub 2011 Dec 2. Tissue Eng Part A. 2012. PMID: 21919798
-
Development of endothelium-denuded human umbilical veins as living scaffolds for tissue-engineered small-calibre vascular grafts.J Tissue Eng Regen Med. 2013 Apr;7(4):324-36. doi: 10.1002/term.529. Epub 2012 Jun 11. J Tissue Eng Regen Med. 2013. PMID: 22689499
-
Scaffolds in vascular regeneration: current status.Vasc Health Risk Manag. 2015 Jan 19;11:79-91. doi: 10.2147/VHRM.S50536. eCollection 2015. Vasc Health Risk Manag. 2015. PMID: 25632236 Free PMC article. Review.
-
Tissue engineering a small diameter vessel substitute: engineering constructs with select biomaterials and cells.Curr Vasc Pharmacol. 2012 May;10(3):347-60. doi: 10.2174/157016112799959378. Curr Vasc Pharmacol. 2012. PMID: 22239637 Review.
Cited by
-
The Tissue-Engineered Vascular Graft-Past, Present, and Future.Tissue Eng Part B Rev. 2016 Feb;22(1):68-100. doi: 10.1089/ten.teb.2015.0100. Epub 2015 Oct 8. Tissue Eng Part B Rev. 2016. PMID: 26447530 Free PMC article.
-
The effect of terminal sterilization on structural and biophysical properties of a decellularized collagen-based scaffold; implications for stem cell adhesion.J Biomed Mater Res B Appl Biomater. 2015 Feb;103(2):397-406. doi: 10.1002/jbm.b.33213. Epub 2014 Jun 3. J Biomed Mater Res B Appl Biomater. 2015. PMID: 24895116 Free PMC article.
-
Silica nanoparticles enhance interfacial self-adherence of a multi-layered extracellular matrix scaffold for vascular tissue regeneration.Biotechnol Lett. 2024 Jun;46(3):469-481. doi: 10.1007/s10529-024-03469-0. Epub 2024 Feb 17. Biotechnol Lett. 2024. PMID: 38368285
-
Tuning scaffold mechanics by laminating native extracellular matrix membranes and effects on early cellular remodeling.J Biomed Mater Res A. 2014 May;102(5):1325-33. doi: 10.1002/jbm.a.34791. Epub 2013 Jun 11. J Biomed Mater Res A. 2014. PMID: 23666819 Free PMC article.
-
Rapid fabrication of reinforced and cell-laden vascular grafts structurally inspired by human coronary arteries.Nat Commun. 2019 Jul 15;10(1):3098. doi: 10.1038/s41467-019-11090-3. Nat Commun. 2019. PMID: 31308369 Free PMC article.
References
-
- Puskas J.E. Chen Y. Biomedical application of commercial polymers and novel polyisobutylene-based thermoplastic elastomers for soft tissue replacement. Biomacromolecules. 2004;5:1141. - PubMed
-
- Gunatillake P. Mayadunne R. Adhikari R. Recent developments in biodegradable synthetic polymers. Biotechnol Annu Rev. 2006;12:301. - PubMed
-
- L'Heureux N. Dusserre N. Marini A. Garrido S. de la Fuente L. McAllister T. Technology insight: the evolution of tissue-engineered vascular grafts—from research to clinical practice. Nat Clin Pract Cardiovasc Med. 2007;4:389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

