A phase 1, randomized, placebo-controlled, dose-escalation study of an anti-IL-13 monoclonal antibody in healthy subjects and mild asthmatics
- PMID: 22616628
- PMCID: PMC3555051
- DOI: 10.1111/j.1365-2125.2012.04334.x
A phase 1, randomized, placebo-controlled, dose-escalation study of an anti-IL-13 monoclonal antibody in healthy subjects and mild asthmatics
Abstract
Aims: IL-13 is implicated as an important mediator of the pathology of asthma. This first clinical study with GSK679586, a novel humanized anti-IL-13 IgG1 monoclonal antibody, evaluated the safety, pharmacokinetics and pharmacodynamics of escalating single and repeat doses of GSK679586.
Methods: In this randomized, double-blind study, healthy subjects received single intravenous infusions of GSK679586 (0.005, 0.05, 0.5, 2.5, 10 mg kg(-1)) or placebo and mild intermittent asthmatics received two once monthly intravenous infusions of GSK679586 (2.5, 10, 20 mg kg(-1)) or placebo.
Results: GSK679586 displayed approximately linear pharmacokinetics (based on AUC and C(max)) with limited accumulation upon repeat administration. In mild intermittent asthmatics, treatment with GSK679586 produced an increase in serum total IL-13 concentrations, indicative of GSK679586-IL-13 complex formation. Additionally, mean levels of exhaled nitric oxide (FeNO), a marker of pulmonary inflammation, were reduced relative to baseline at 2.5, 10 and 20 mg kg(-1) doses of GSK679586 at both 2 weeks (19%, 44% and 52% decreases) and 8 weeks (29%, 55% and 42% decreases) after the second infusion. GSK679586 was well tolerated; the incidence of AEs was comparable across all presumed biologically active doses and there were no treatment-related SAEs.
Conclusions: GSK679586 demonstrated dose-dependent pharmacological activity in the lungs of mild intermittent asthmatics. These findings, together with the favourable safety profile and advantageous PK characteristics of a monoclonal antibody (e.g. a long half-life supporting less frequent dosing), warrant further investigation of GSK679586 in a broader asthma patient population.
Trial registration: ClinicalTrials.gov NCT00411814.
© 2012 The Authors. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures
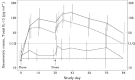
 , 20 mg kg–1 (n = 9);
, 20 mg kg–1 (n = 9);  , 10 mg kg–1 (n = 6);
, 10 mg kg–1 (n = 6);  , 2.5 mg kg–1 (n = 6);
, 2.5 mg kg–1 (n = 6);  , placebo (n = 7). LLQ lower limit of quantification (30.86 pg ml–1). Samples with total IL-13 below LLQ were imputed as ½ of LLQ (15.43 pg ml–1)
, placebo (n = 7). LLQ lower limit of quantification (30.86 pg ml–1). Samples with total IL-13 below LLQ were imputed as ½ of LLQ (15.43 pg ml–1)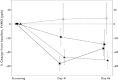
 , placebo;
, placebo;  , 2.5 mg kg–1;
, 2.5 mg kg–1;  , 10 mg kg–1;
, 10 mg kg–1;  , 20 mg kg–1
, 20 mg kg–1References
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Woolley KL, Adelroth E, Woolley MJ, Ellis R, Jordana M, O'Byrne PM. Granulocyte-macrophage colony-stimulating factor, eosinophils and eosinophil-cationic protein in subjects with and without mild, stable, atopic asthma. Eur Respir J. 1994;7:1576–84. - PubMed
-
- Heinig HA, Boulet LP, Croonenborghs L, Möllers MJ. The effect of high-dose fluticasone proprionate and budesonide on lung function and asthma exacerbations in patients with severe asthma. Respir Med. 1999;93:613–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

