Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust
- PMID: 22616633
- PMCID: PMC3429863
- DOI: 10.1111/j.1462-2920.2012.02778.x
Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust
Abstract
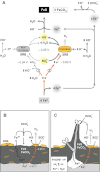
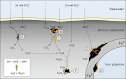
Iron (Fe(0) ) corrosion in anoxic environments (e.g. inside pipelines), a process entailing considerable economic costs, is largely influenced by microorganisms, in particular sulfate-reducing bacteria (SRB). The process is characterized by formation of black crusts and metal pitting. The mechanism is usually explained by the corrosiveness of formed H(2) S, and scavenge of 'cathodic' H(2) from chemical reaction of Fe(0) with H(2) O. Here we studied peculiar marine SRB that grew lithotrophically with metallic iron as the only electron donor. They degraded up to 72% of iron coupons (10 mm × 10 mm × 1 mm) within five months, which is a technologically highly relevant corrosion rate (0.7 mm Fe(0) year(-1) ), while conventional H(2) -scavenging control strains were not corrosive. The black, hard mineral crust (FeS, FeCO(3) , Mg/CaCO(3) ) deposited on the corroding metal exhibited electrical conductivity (50 S m(-1) ). This was sufficient to explain the corrosion rate by electron flow from the metal (4Fe(0) → 4Fe(2+) + 8e(-) ) through semiconductive sulfides to the crust-colonizing cells reducing sulfate (8e(-) + SO(4) (2-) + 9H(+) → HS(-) + 4H(2) O). Hence, anaerobic microbial iron corrosion obviously bypasses H(2) rather than depends on it. SRB with such corrosive potential were revealed at naturally high numbers at a coastal marine sediment site. Iron coupons buried there were corroded and covered by the characteristic mineral crust. It is speculated that anaerobic biocorrosion is due to the promiscuous use of an ecophysiologically relevant catabolic trait for uptake of external electrons from abiotic or biotic sources in sediments.
© 2012 Society for Applied Microbiology and Blackwell Publishing Ltd.
Figures




References
-
- Araujo JC, Teran FC, Oliveira RA, Nour EAA, Montenegro MAP, Campos JR, Vazoller RF. Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J Electron Microsc (Tokyo) 2003;52:429–433. - PubMed
-
- Badziong W, Thauer RK. Growth yields and growth rates of Desulfovibrio vulgaris (Marburg) growing on hydrogen plus sulfate and hydrogen plus thiosulfate as sole energy sources. Arch Microbiol. 1978;117:209–214. - PubMed
-
- Beech IB, Sunner IA. Sulphate-reducing bacteria and their role in corrosion of ferrous materials. In: Barton LL, Hamilton WA, editors. Sulphate-Reducing Bacteria: Environmental and Engineered Systems. Cambridge, UK: Cambridge University Press; 2007. pp. 459–482.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

