Post-translational regulation of TGF-β receptor and Smad signaling
- PMID: 22617150
- PMCID: PMC4240271
- DOI: 10.1016/j.febslet.2012.05.010
Post-translational regulation of TGF-β receptor and Smad signaling
Abstract
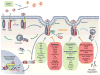
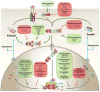
TGF-β family signaling through Smads is conceptually a simple and linear signaling pathway, driven by sequential phosphorylation, with type II receptors activating type I receptors, which in turn activate R-Smads. Nevertheless, TGF-β family proteins induce highly complex programs of gene expression responses that are extensively regulated, and depend on the physiological context of the cells. Regulation of TGF-β signaling occurs at multiple levels, including TGF-β activation, formation, activation and destruction of functional TGF-β receptor complexes, activation and degradation of Smads, and formation of Smad transcription complexes at regulatory gene sequences that cooperate with a diverse set of DNA binding transcription factors and coregulators. Here we discuss recent insights into the roles of post-translational modifications and molecular interaction networks in the functions of receptors and Smads in TGF-β signal responses. These layers of regulation demonstrate how a simple signaling system can be coopted to exert exquisitely regulated, complex responses.
Copyright © 2012 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
TGF-beta signaling by Smad proteins.Adv Immunol. 2000;75:115-57. doi: 10.1016/s0065-2776(00)75003-6. Adv Immunol. 2000. PMID: 10879283 Review.
-
Negative regulation of TGF-beta receptor/Smad signal transduction.Curr Opin Cell Biol. 2007 Apr;19(2):176-84. doi: 10.1016/j.ceb.2007.02.015. Epub 2007 Feb 20. Curr Opin Cell Biol. 2007. PMID: 17317136 Review.
-
Posttranslational Regulation of Smads.Cold Spring Harb Perspect Biol. 2016 Dec 1;8(12):a022087. doi: 10.1101/cshperspect.a022087. Cold Spring Harb Perspect Biol. 2016. PMID: 27908935 Free PMC article. Review.
-
How the Smads regulate transcription.Int J Biochem Cell Biol. 2008;40(3):383-408. doi: 10.1016/j.biocel.2007.09.006. Epub 2007 Oct 7. Int J Biochem Cell Biol. 2008. PMID: 18061509 Review.
-
Transforming Growth Factor-β Receptors and Smads: Regulatory Complexity and Functional Versatility.Trends Cell Biol. 2017 Sep;27(9):658-672. doi: 10.1016/j.tcb.2017.04.005. Epub 2017 May 25. Trends Cell Biol. 2017. PMID: 28552280 Review.
Cited by
-
TGF-β Signaling from Receptors to Smads.Cold Spring Harb Perspect Biol. 2016 Sep 1;8(9):a022061. doi: 10.1101/cshperspect.a022061. Cold Spring Harb Perspect Biol. 2016. PMID: 27449815 Free PMC article. Review.
-
Dysregulated Inflammatory Signaling upon Charcot-Marie-Tooth Type 1C Mutation of SIMPLE Protein.Mol Cell Biol. 2015 Jul;35(14):2464-78. doi: 10.1128/MCB.00300-15. Mol Cell Biol. 2015. PMID: 25963657 Free PMC article.
-
TSPAN4-positive migrasome derived from retinal pigmented epithelium cells contributes to the development of proliferative vitreoretinopathy.J Nanobiotechnology. 2022 Dec 9;20(1):519. doi: 10.1186/s12951-022-01732-y. J Nanobiotechnology. 2022. PMID: 36494806 Free PMC article.
-
Specification of BMP Signaling.Cells. 2019 Dec 5;8(12):1579. doi: 10.3390/cells8121579. Cells. 2019. PMID: 31817503 Free PMC article. Review.
-
The Role of BMP Signaling in Endothelial Heterogeneity.Front Cell Dev Biol. 2021 Jun 21;9:673396. doi: 10.3389/fcell.2021.673396. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34235147 Free PMC article. Review.
References
-
- Derynck R, Miyazono K. TGF-β and the TGF-β Family. In: Derynck R, Miyazono K, editors. The TGF-β Family. Cold Spring Harbor Laboratory Press; 2008. pp. 29–44.
-
- Derynck R, Miyazono K, editors. The TGF-β Family. Cold Spring Harbor Laboratory Press; 2008.
-
- Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massague J. The TGF β receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell. 2001;8:671–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

