Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches
- PMID: 22617385
- PMCID: PMC3383588
- DOI: 10.4161/cc.20437
Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches
Abstract
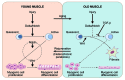
Aging is unmistakable and undeniable in mammals. Interestingly, mice develop cataracts, muscle atrophy, osteoporosis, obesity, diabetes and cognitive deficits after just 2-3 postnatal years, while it takes seven or more decades for the same age-specific phenotypes to develop in humans. Thus, chronological age corresponds differently with biological age in metazoan species and although many theories exist, we do not understand what controls the rate of mammalian aging. One interesting idea is that species-specific rate of aging represents a ratio of tissue attrition to tissue regeneration. Furthermore, current findings suggest that the age-imposed biochemical changes in the niches of tissue stem cells inhibit performance of this regenerative pool, which leads to the decline of tissue maintenance and repair. If true, slowing down stem cell and niche aging, thereby promoting tissue regeneration, could slow down the process of tissue and organismal aging. In this regard, recent studies of heterochronic parabiosis provide important clues as to the mechanisms of stem cell aging and suggest novel strategies for enhancing tissue repair in the old. Here we review current literature on the relationship between the vigor of tissue stem cells and the process of aging, with an emphasis on the rejuvenation of old tissues by the extrinsic modifications of stem cell niches.
Figures

References
-
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
