Continuous compared with cyclic oral contraceptives for the treatment of primary dysmenorrhea: a randomized controlled trial
- PMID: 22617578
- PMCID: PMC3631421
- DOI: 10.1097/AOG.0b013e318257217a
Continuous compared with cyclic oral contraceptives for the treatment of primary dysmenorrhea: a randomized controlled trial
Abstract
Objective: To estimate whether continuous oral contraceptive pills (OCPs) will result in more pain relief in primary dysmenorrhea patients than cyclic OCPs, which induce withdrawal bleeding with associated pain and symptoms.
Methods: We conducted a double-blind, randomized, controlled trial comparing continuous to a cyclic 21-7 OCP regimen (gestodene 0.075 mg and ethinyl estradiol 20 microgram) for 6 months in 38 primary dysmenorrhea patients. The primary outcome was the difference in subjective perception of pain as measured by the visual analog scale over a period of 6 months.
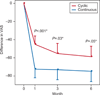
Results: Twenty-nine patients completed the study. In both groups, pain reduction measured by visual analog scale declined over time and was significant at 6 months compared with baseline, with no difference between groups. Continuous regimen was superior to cyclic regimen after 1 month (mean difference -27.3, 95% confidence interval [CI] -40.5 to -14.2; P<.001) and 3 months (mean difference -17.8, 95% CI -33.4 to -2.1; P=.03) of treatment. Secondary outcomes noted no difference between groups in terms of menstrual distress as measured by the Moos Menstrual Distress Questionnaire. After 6 months, there was an increase in weight and a decrease in systolic blood pressure in the continuous group compared with the cyclic group.
Conclusion: Both regimens of OCPs are effective in the treatment of primary dysmenorrhea. Continuous OCPs outperform cyclic OCPs in the short term, but this difference is lost after 6 months.
Clinical trial registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00517556.
Level of evidence: I.
Figures
References
-
- Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144(6):655–660. - PubMed
-
- Dawood MY. Ibuprofen and dysmenorrhea. Am J Med. 1984;77(1A):87–94. - PubMed
-
- Hauksson A, Akerlund M, Melin P. Uterine blood flow and myometrial activity at menstruation, and the action of vasopressin and a synthetic antagonist. Br J Obstet Gynaecol. 1988;95(9):898–904. - PubMed
-
- Pulkkinen MO. Prostaglandins and the non-pregnant uterus. The pathophysiology of primary dysmenorrhea. Acta Obstet Gynecol Scand Suppl. 1983;113:63–67. - PubMed
-
- Akerlund M. Vascularization of human endometrium. Uterine blood flow in healthy condition and in primary dysmenorrhoea. Ann N Y Acad Sci. 1994;734:47–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials