Streptococcus and rheumatic fever
- PMID: 22617826
- PMCID: PMC3645882
- DOI: 10.1097/BOR.0b013e32835461d3
Streptococcus and rheumatic fever
Abstract
Purpose of review: To give an overview of the current hypotheses of the pathogenesis of rheumatic fever and group A streptococcal autoimmune sequelae of the heart valve and brain.
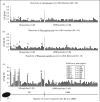
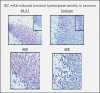
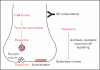
Recent findings: Human monoclonal antibodies (mAbs) derived from rheumatic heart disease have provided evidence for crossreactive autoantibodies that target the dominant group A streptococcal epitope of the group A carbohydrate, N-acetyl-beta-D-glucosamine (GlcNAc), and heart valve endothelium, laminin and laminar basement membrane. T cells in peripheral blood and in rheumatic heart valves revealed the presence of T cells crossreactive with streptococcal M protein and cardiac myosin. For initiation of disease, evidence suggests a two-hit hypothesis for antibody attack on the valve endothelium with subsequent extravasation of T cells through activated endothelium into the valve to form granulomatous lesions and Aschoff bodies. Autoantibodies against the group A streptococcal carbohydrate epitope GlcNAc and cardiac myosin and its peptides appear during progression of rheumatic heart disease. However, autoantibodies against collagen that are not crossreactive may form because of the release of collagen from damaged valve or to responses to collagen bound in vitro by certain serotypes of streptococci. In Sydenham chorea, human mAbs derived from disease target the group A carbohydrate epitope GlcNAc and gangliosides and dopamine receptors found on the surface of neuronal cells in the brain. Human mAbs and autoantibodies in Sydenham chorea were found to signal neuronal cells and activate calcium calmodulin-dependent protein kinase II (CaMKII) in neuronal cells and recognize the intracellular protein biomarker tubulin.
Summary: To summarize, pathogenic mechanisms of crossreactive autoantibodies which target the valve in rheumatic heart disease and the neuronal cell in Sydenham chorea share a common streptococcal epitope GlcNAc and target intracellular biomarkers of disease including cardiac myosin in the myocardium and tubulin, a protein abundant in the brain. However, intracellular antigens are not believed to be the basis for disease. The theme of molecular mimicry in streptococcal autoimmune sequelae is the recognition of targeted intracellular biomarker antigens such as cardiac myosin and brain tubulin, while targeting extracellular membrane antigens such as laminin on the valve surface endothelium or lysoganglioside and dopamine receptors in the brain. Antibody binding to these cell surface antigens may lead to valve damage in rheumatic heart disease or neuropsychiatric behaviors and involuntary movements in Sydenham chorea.
Figures


References
-
- Stollerman GH, editor. Rheumatic fever and streptococcal infection. Grune and Stratton; New York: 1975. pp. 1–145.
-
- Taranta A, Stollerman GH. The relationship of Sydenham's chorea to infection with group A streptococci. Am J Med. 1956:170–175. - PubMed
-
- Stollerman GH. Rheumatic fever. Lancet. 1997;349:935–942. - PubMed
-
- Dajani AS. Guidelines for the diagnosis of rheumatic fever (Jones criteria 1992 update). J Am Med Assoc 1992. 268:2069–2073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

