Immunogenicity and safety of measles-mumps-rubella and varicella vaccines coadministered with a fourth dose of Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine in toddlers: a pooled analysis of randomized trials
- PMID: 22617844
- PMCID: PMC3551873
- DOI: 10.4161/hv.20357
Immunogenicity and safety of measles-mumps-rubella and varicella vaccines coadministered with a fourth dose of Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine in toddlers: a pooled analysis of randomized trials
Abstract
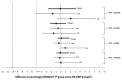
A pooled analysis was conducted of 1257 toddlers who received a fourth dose of Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine (HibMenCY-TT) or Hib conjugate vaccine (Hib polysaccharide conjugated to N. meningitidis outer membrane protein) coadministered with measles-mumps-rubella (MMR) and varicella (VAR) vaccines (NCT00134719/NCT00289783). Noninferiority of immunological responses to MMR and VAR was demonstrated between groups and incidences of MMR- and VAR-specific solicited symptoms were similar, indicating that HibMenCY-TT can be coadministered with MMR and VAR.
Figures
References
-
- Nolan T, Lambert S, Roberton D, Marshall H, Richmond P, Streeton C, et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine. 2007;25:8487–99. doi: 10.1016/j.vaccine.2007.10.013. - DOI - PubMed
-
- Habermehl P, Leroux-Roels G, Sänger R, Mächler G, Boutriau D. Combined Haemophilus influenzae type b and Neisseria meningitidis serogroup C (HibMenC) or serogroup C and Y-tetanus toxoid conjugate (and HibMenCY) vaccines are well-tolerated and immunogenic when administered according to the 2,3,4 months schedule with a fourth dose at 12-18 months of age. Hum Vaccin. 2010;6:640–51. doi: 10.4161/hv.6.8.12154. - DOI - PubMed
-
- Marchant CD, Miller JM, Marshall GS, Blatter M, Aris E, Friedland LR, et al. HibMenCY-TT 005 Study Group Randomized trial to assess immunogenicity and safety of Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine in infants. Pediatr Infect Dis J. 2010;29:48–52. doi: 10.1097/INF.0b013e3181c3ce88. - DOI - PubMed
-
- Nolan T, Richmond P, Marshall H, McVernon J, Alexander K, Mesaros N, et al. Immunogenicity and safety of an investigational combined haemophilus influenzae type B-Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190–6. doi: 10.1097/INF.0b013e3181fcb2bf. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
