Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke
- PMID: 22618383
- PMCID: PMC3404219
- DOI: 10.1161/STROKEAHA.111.646224
Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke
Abstract
Background and purpose: Axonal remodeling is critical to brain repair after stroke. The present study investigated axonal outgrowth after stroke and the signaling pathways mediating axonal outgrowth in cortical neurons.
Methods: Using a rodent model of middle cerebral artery occlusion, we examined high-molecular weight neurofilament (NFH) immunoreactive axons and myelin basic protein-positive oligodendrocytes in the peri-infarct area. In vitro, using cultured cortical neurons in a microfluidic chamber challenged by oxygen-glucose deprivation (OGD), we investigated mechanisms selectively regulating axonal outgrowth after OGD.
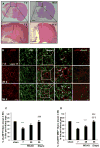
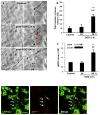
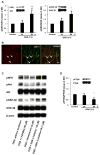
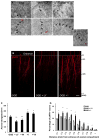
Results: NFH(+) axons and MBP(+) oligodendrocytes substantially increased in the peri-infarct area during stroke recovery, concomitantly with an increase in dendrites and spines identified by Golgi-Cox staining. In vitro, cortical neurons subjected to OGD exhibited significant increases in axonal outgrowth and in phosphorylated NFH protein levels, concurrently with downregulation of phosphatase tensin homolog deleted on chromosome 10, activation of Akt, and inactivation of glycogen synthase kinase-3β in regenerated axons. Blockage of phosphoinositide 3-kinase with pharmacological inhibitors suppressed Akt activation and attenuated phosphorylation of glycogen synthase kinase-3β, which resulted in suppression of phosphorylated NFH and axonal outgrowth after OGD; whereas GSK-3 inhibitors augmented axonal regeneration and elevated phosphorylated NFH levels after OGD.
Conclusions: Stroke induces axonal outgrowth and myelination in rodent ischemic brain during stroke recovery, and the phosphoinositide 3-kinase/Akt/glycogen synthase kinase-3β signaling pathway mediates axonal regeneration of cortical neurons after OGD.
Conflict of interest statement
Conflict of interest: None
Figures

References
-
- Julien JP, Mushynski WE. Multiple phosphorylation sites in mammalian neurofilament polypeptides. J Biol Chem. 1982;257:10467–10470. - PubMed
-
- de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating schwann cells. Cell. 1992;68:451–463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

