Intrastriatal B-cell administration limits infarct size after stroke in B-cell deficient mice
- PMID: 22618587
- PMCID: PMC3427715
- DOI: 10.1007/s11011-012-9317-7
Intrastriatal B-cell administration limits infarct size after stroke in B-cell deficient mice
Abstract
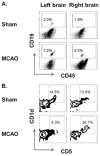
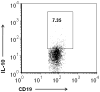
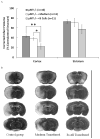
Recent evidence emphasizes B-cells as a major regulatory cell type that plays an important role in limiting the pathogenic effects of ischemic stroke. The aim of the current study was to extend this initial observation to specifically examine the infiltration of regulatory B-cells and to determine if the effect of B-cells to limit the inflammatory response to cerebral ischemia is mediated by their action centrally or peripherally. Our data demonstrate the increased presence of a regulatory B-cell subset in the affected hemisphere of wild-type mice after middle cerebral artery occlusion (MCAO). We further explored the use of a novel method of stereotaxic cell delivery to bypass the blood brain barrier (BBB) and introduce CD19(+) B-cells directly into the striatum as compared to peripheral administration of B-cells. Infarct volumes after 60 minutes of MCAO and 48 hours of reperfusion were determined in B-cell deficient μMT( -/- ) mice with and without replacement of either B-cells or medium. Infarct size was significantly decreased in cerebral cortex after intrastriatal transfer of 100,000 B-cells to μMT(-/-) mice vs. controls, with a comparable effect on infarct size as obtained by 50 million B-cells transferred intraperitoneally. These findings support the hypothesis that B-cells play a protective role against ischemic brain injury, and suggest that B-cells may serve as a novel therapeutic agent for modulating the immune response in central nervous system inflammation after stroke.
Figures

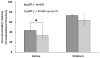
References
-
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;22 (2):586–592. - PubMed
-
- Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. Journal of Immunology. 2011;186:5569–5579. - PubMed
-
- de Bilbao FDA, Moll T, Garcia-Gabay I, Vallet P, Langhans W, Giannakopoulos P. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. Journal of Neurochemistry. 2009;110:12–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

