The role of glutamine synthetase and glutamate dehydrogenase in cerebral ammonia homeostasis
- PMID: 22618691
- PMCID: PMC3490058
- DOI: 10.1007/s11064-012-0803-4
The role of glutamine synthetase and glutamate dehydrogenase in cerebral ammonia homeostasis
Abstract
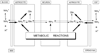
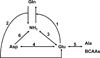
In the brain, glutamine synthetase (GS), which is located predominantly in astrocytes, is largely responsible for the removal of both blood-derived and metabolically generated ammonia. Thus, studies with [(13)N]ammonia have shown that about 25 % of blood-derived ammonia is removed in a single pass through the rat brain and that this ammonia is incorporated primarily into glutamine (amide) in astrocytes. Major pathways for cerebral ammonia generation include the glutaminase reaction and the glutamate dehydrogenase (GDH) reaction. The equilibrium position of the GDH-catalyzed reaction in vitro favors reductive amination of α-ketoglutarate at pH 7.4. Nevertheless, only a small amount of label derived from [(13)N]ammonia in rat brain is incorporated into glutamate and the α-amine of glutamine in vivo. Most likely the cerebral GDH reaction is drawn normally in the direction of glutamate oxidation (ammonia production) by rapid removal of ammonia as glutamine. Linkage of glutamate/α-ketoglutarate-utilizing aminotransferases with the GDH reaction channels excess amino acid nitrogen toward ammonia for glutamine synthesis. At high ammonia levels and/or when GS is inhibited the GDH reaction coupled with glutamate/α-ketoglutarate-linked aminotransferases may, however, promote the flow of ammonia nitrogen toward synthesis of amino acids. Preliminary evidence suggests an important role for the purine nucleotide cycle (PNC) as an additional source of ammonia in neurons (Net reaction: L-Aspartate + GTP + H(2)O → Fumarate + GDP + P(i) + NH(3)) and in the beat cycle of ependyma cilia. The link of the PNC to aminotransferases and GDH/GS and its role in cerebral nitrogen metabolism under both normal and pathological (e.g. hyperammonemic encephalopathy) conditions should be a productive area for future research.
Figures

References
-
- Berl S, Takagaki G, Clarke DD, Waelsch H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem. 1962;237:2562–2569. - PubMed
-
- Gebhardt R, Ebert A, Bauer G. Heterogeneous expression of glutamine synthetase mRNA in rat liver parenchyma revealed by in situ hybridization and Northern blot analysis of RNA from periportal and perivenous hepatocytes. FEBS Lett. 1988;241:89–93. - PubMed
-
- Brosnan ME, Brosnan JT. Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr. 2009;90:857S–861S. - PubMed
-
- Berl S, Takagaki G, Clarke DD, Waelsch H. Carbon dioxide fixation in the brain. J Biol Chem. 1962;237:2570–2573. - PubMed
-
- Lapidot A, Gopher A. Cerebral metabolic compartmentation. Estimation of glucose flux via pyruvate carboxylase/pyruvate dehydrogenase by 13C NMR isotopomer analysis of D-[U-13C]glucose metabolites. J Biol Chem. 1994;269:27198–27208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

