Direct comparison of small RNA and transcription factor signaling
- PMID: 22618873
- PMCID: PMC3424570
- DOI: 10.1093/nar/gks439
Direct comparison of small RNA and transcription factor signaling
Abstract
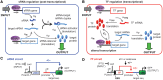
Small RNAs (sRNAs) and proteins acting as transcription factors (TFs) are the principal components of gene networks. These two classes of signaling molecules have distinct mechanisms of action; sRNAs control mRNA translation, whereas TFs control mRNA transcription. Here, we directly compare the properties of sRNA and TF signaling using mathematical models and synthetic gene circuits in Escherichia coli. We show the abilities of sRNAs to act on existing target mRNAs (as opposed to TFs, which alter the production of future target mRNAs) and, without needing to be first translated, have surprisingly little impact on the dynamics. Instead, the dynamics are primarily determined by the clearance rates, steady-state concentrations and response curves of the sRNAs and TFs; these factors determine the time delay before a target gene's expression can maximally respond to changes in sRNA and TF transcription. The findings are broadly applicable to the analysis of signaling in gene networks, and we demonstrate that they can be used to rationally reprogram the dynamics of synthetic circuits.
Figures

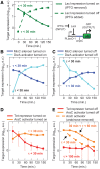
 , where α is the maximum expression due to induction (units: fluorescence, a.u.), TF* is the TF transcription rate which is presumed to be proportional to the TF concentration (units: relative transcription, a.u.), K is the TF transcription rate that results in half the maximal level of induced expression (units: relative transcription, a.u.), n is the Hill coefficient (unitless) and c is the level of expression before IPTG is added (units: fluorescence, a.u.). The fit values for AraC-rpoS are: R2 = 0.98, reduced χ2 = 44.9, α = 3.43 ± 0.61 a.u., K = 0.34 ± 0.17 a.u., n = 0.57 ± 0.10, c = 0.99 ± 0.08 a.u. The fit values for AraC-ompC are: R2 > 0.99, reduced χ2 = 3.24, α = 7.47 ± 0.46 a.u., K = 0.05 ± 0.004 a.u., n = 0.81 ± 0.08, c = 0.55 ± 0.21 a.u.
, where α is the maximum expression due to induction (units: fluorescence, a.u.), TF* is the TF transcription rate which is presumed to be proportional to the TF concentration (units: relative transcription, a.u.), K is the TF transcription rate that results in half the maximal level of induced expression (units: relative transcription, a.u.), n is the Hill coefficient (unitless) and c is the level of expression before IPTG is added (units: fluorescence, a.u.). The fit values for AraC-rpoS are: R2 = 0.98, reduced χ2 = 44.9, α = 3.43 ± 0.61 a.u., K = 0.34 ± 0.17 a.u., n = 0.57 ± 0.10, c = 0.99 ± 0.08 a.u. The fit values for AraC-ompC are: R2 > 0.99, reduced χ2 = 3.24, α = 7.47 ± 0.46 a.u., K = 0.05 ± 0.004 a.u., n = 0.81 ± 0.08, c = 0.55 ± 0.21 a.u.

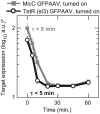
References
-
- Repoila F, Darfeuille F. Small regulatory non-coding RNAs in bacteria: physiology and mechanistic aspects. Biol. Cell. 2009;101:117–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

