An intravaginal ring for the simultaneous delivery of multiple drugs
- PMID: 22619076
- PMCID: PMC3857731
- DOI: 10.1002/jps.23208
An intravaginal ring for the simultaneous delivery of multiple drugs
Abstract
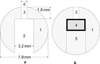
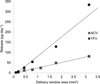
Intravaginal delivery of microbicide combinations is a promising approach for the prevention of sexually transmitted infections, but requires a method of providing simultaneous, independent release of multiple agents into the vaginal compartment. A novel intravaginal ring (IVR) platform has been developed for simultaneous delivery of the reverse-transcriptase inhibitor tenofovir (TFV) and the guanosine analogue antiviral acyclovir (ACV) with independent control of release rate for each drug. The IVR is based on a pod design, with up to 10 individual polymer-coated drug cores embedded in the ring releasing through preformed delivery channels. The release rate from each pod is controlled independently of the others by the drug properties, polymer coating, and size and number of delivery channels. Pseudo-zero-order in vitro release of TFV (144 ± 10 µg day) and ACV (120 ± 19 µg day⁻¹) from an IVR containing both drugs was sustained for 28 days. The mechanical properties of the pod IVR were evaluated and compared with the commercially available Estring® (Pfizer, NY, NY). The pod-IVR design enables the vaginal delivery of multiple microbicides with differing physicochemical properties, and is an attractive approach for the sustained intravaginal delivery of relatively hydrophilic drugs that are difficult to deliver using conventional matrix IVR technology.
Copyright © 2012 Wiley Periodicals, Inc.
Figures




Similar articles
-
Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring.Antimicrob Agents Chemother. 2012 Feb;56(2):875-82. doi: 10.1128/AAC.05662-11. Epub 2011 Nov 28. Antimicrob Agents Chemother. 2012. PMID: 22123689 Free PMC article.
-
Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques.Antimicrob Agents Chemother. 2012 Nov;56(11):5952-60. doi: 10.1128/AAC.01198-12. Epub 2012 Sep 10. Antimicrob Agents Chemother. 2012. PMID: 22964245 Free PMC article.
-
Pharmacokinetics and preliminary safety study of pod-intravaginal rings delivering antiretroviral combinations for HIV prophylaxis in a macaque model.Antimicrob Agents Chemother. 2014 Sep;58(9):5125-35. doi: 10.1128/AAC.02871-14. Epub 2014 Jun 16. Antimicrob Agents Chemother. 2014. PMID: 24936594 Free PMC article.
-
Drug delivery in multiple indication (multipurpose) prevention technologies: systems to prevent HIV-1 transmission and unintended pregnancies or HSV-2 transmission.Expert Opin Drug Deliv. 2012 Apr;9(4):417-27. doi: 10.1517/17425247.2012.668183. Epub 2012 Mar 2. Expert Opin Drug Deliv. 2012. PMID: 22385316 Review.
-
Topical microbicides for preventing sexually transmitted infections.Cochrane Database Syst Rev. 2021 Mar 13;3(3):CD007961. doi: 10.1002/14651858.CD007961.pub3. Cochrane Database Syst Rev. 2021. PMID: 33719075 Free PMC article.
Cited by
-
Phase I trial of pod-intravaginal rings delivering antiretroviral agents for HIV-1 prevention: Rectal drug exposure from vaginal dosing with tenofovir disoproxil fumarate, emtricitabine, and maraviroc.PLoS One. 2018 Aug 22;13(8):e0201952. doi: 10.1371/journal.pone.0201952. eCollection 2018. PLoS One. 2018. PMID: 30133534 Free PMC article. Clinical Trial.
-
Innovations in monoclonal antibody-based multipurpose prevention technology (MPT) for the prevention of sexually transmitted infections and unintended pregnancy.Front Reprod Health. 2024 Jan 9;5:1337479. doi: 10.3389/frph.2023.1337479. eCollection 2023. Front Reprod Health. 2024. PMID: 38264184 Free PMC article. Review.
-
Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: from past failures to future hopes.Drug Des Devel Ther. 2017 Jun 15;11:1767-1787. doi: 10.2147/DDDT.S133170. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28670111 Free PMC article. Review.
-
In search of the optimal delivery method for anti-HIV microbicides: are intravaginal rings the way forward?Expert Rev Anti Infect Ther. 2013 Jan;11(1):5-8. doi: 10.1586/eri.12.155. Expert Rev Anti Infect Ther. 2013. PMID: 23428097 Free PMC article. No abstract available.
-
Use of simulated vaginal and menstrual fluids to model in vivo discolouration of silicone elastomer vaginal rings.Int J Pharm X. 2021 May 1;3:100081. doi: 10.1016/j.ijpx.2021.100081. eCollection 2021 Dec. Int J Pharm X. 2021. PMID: 34027386 Free PMC article.
References
-
- Geneva, Switzerland: World Health Organization; 2011. [Accessed April 2, 2012]. Global HIV/AIDS response: Epidemic update and health sector progress towards universal access: Progress report 2011. at: http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf.
-
- Shattock RJ, Warren M, McCormack S, Hankins CA. Turning the tide against HIV. Science. 2011;333(6038):42–43. - PubMed
-
- D’Cruz OJ, Uckun FM. Clinical development of microbicides for the prevention of HIV infection. Curr Pharm Des. 2004;10(3):315–336. - PubMed
-
- Karim QA, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. - PMC - PubMed
-
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang FR, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

