A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells
- PMID: 22619321
- PMCID: PMC3387091
- DOI: 10.1073/pnas.1120534109
A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells
Abstract
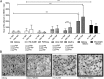
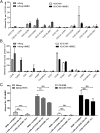
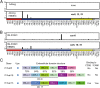
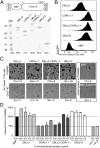
Cerebral malaria (CM) is a deadly complication of Plasmodium falciparum infection, but specific interactions involved in cerebral homing of infected erythrocytes (IEs) are poorly understood. In this study, P. falciparum-IEs were characterized for binding to primary human brain microvascular endothelial cells (HBMECs). Before selection, CD36 or ICAM-1-binding parasites exhibited punctate binding to a subpopulation of HBMECs and binding was CD36 dependent. Panning of IEs on HBMECs led to a more dispersed binding phenotype and the selection of three var genes, including two that encode the tandem domain cassette 8 (DC8) and were non-CD36 binders. Multiple domains in the DC8 cassette bound to brain endothelium and the cysteine-rich interdomain region 1 inhibited binding of P. falciparum-IEs by 50%, highlighting a key role for the DC8 cassette in cerebral binding. It is mysterious how deadly binding variants are maintained in the parasite population. Clonal parasite lines expressing the two brain-adherent DC8-var genes did not bind to any of the known microvascular receptors, indicating unique receptors are involved in cerebral binding. They could also adhere to brain, lung, dermis, and heart endothelial cells, suggesting cerebral binding variants may have alternative sequestration sites. Furthermore, young African children with CM or nonsevere control cases had antibodies to HBMEC-selected parasites, indicating they had been exposed to related variants during childhood infections. This analysis shows that specific P. falciparum erythrocyte membrane protein 1 types are linked to cerebral binding and suggests a potential mechanism by which individuals may build up immunity to severe disease, in the absence of CM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Molecular basis of severe malaria.Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10130-1. doi: 10.1073/pnas.1207174109. Epub 2012 Jun 7. Proc Natl Acad Sci U S A. 2012. PMID: 22679282 Free PMC article. No abstract available.
References
-
- Baruch DI, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. - PubMed
-
- Su XZ, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. - PubMed
-
- Biggs BA, et al. Adherence of infected erythrocytes to venular endothelium selects for antigenic variants of Plasmodium falciparum. J Immunol. 1992;149:2047–2054. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

