Spike timing plays a key role in synapse elimination at the neuromuscular junction
- PMID: 22619332
- PMCID: PMC3382556
- DOI: 10.1073/pnas.1201147109
Spike timing plays a key role in synapse elimination at the neuromuscular junction
Abstract
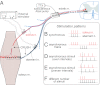
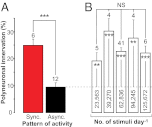
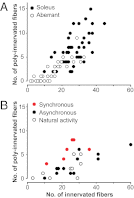
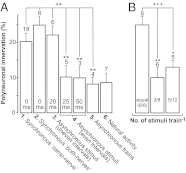
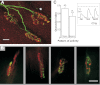
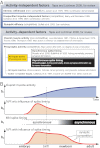
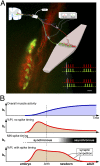
Nerve impulse activity produces both developmental and adult plastic changes in neural networks. For development, however, its precise role and the mechanisms involved remain elusive. Using the classic model of synapse competition and elimination at newly formed neuromuscular junctions, we asked whether spike timing is the instructive signal at inputs competing for synaptic space. Using a rat strain whose soleus muscle is innervated by two nerves, we chronically evoked different temporal spike patterns in the two nerves during synapse formation in the adult. We found that asynchronous activity imposed upon the two nerves promotes synapse elimination, provided that their relative spikes are separated by 25 ms or more; remarkably, this elimination occurs even though an equal number of spikes were evoked in the competing axons. On the other hand, when spikes are separated by 20 ms or less, activity is perceived as synchronous, and elimination is prevented. Thus, in development, as in adult plasticity, precise spike timing plays an instructive role in synaptic modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pre- and post-synaptic roles of action potential activity in synapse elimination revealed by using ectopic neuromuscular junction formation by a foreign nerve.Neurosci Lett. 2020 Mar 23;722:134835. doi: 10.1016/j.neulet.2020.134835. Epub 2020 Feb 10. Neurosci Lett. 2020. PMID: 32057925 Review.
-
Synapse formation and elimination: role of activity studied in different models of adult muscle reinnervation.J Neurosci Res. 2007 Sep;85(12):2610-9. doi: 10.1002/jnr.21143. J Neurosci Res. 2007. PMID: 17139683
-
The timing of impulse activity shapes the process of synaptic competition at the neuromuscular junction.Neuroscience. 2010 May 5;167(2):343-53. doi: 10.1016/j.neuroscience.2010.01.055. Epub 2010 Feb 1. Neuroscience. 2010. PMID: 20132871
-
On the mechanism of action of muscle fibre activity in synapse competition and elimination at the mammalian neuromuscular junction.Eur J Neurosci. 2009 Jun;29(12):2327-34. doi: 10.1111/j.1460-9568.2009.06779.x. Epub 2009 May 29. Eur J Neurosci. 2009. PMID: 19490025
-
Activity-dependent synaptic competition at mammalian neuromuscular junctions.News Physiol Sci. 2004 Jun;19:85-91. doi: 10.1152/nips.01464.2003. News Physiol Sci. 2004. PMID: 15143199 Review.
Cited by
-
Lesson from the neuromuscular junction: role of pattern and timing of nerve activity in synaptic development.Neural Regen Res. 2015 May;10(5):686-8. doi: 10.4103/1673-5374.156944. Neural Regen Res. 2015. PMID: 26109931 Free PMC article. No abstract available.
-
Ultrafast synaptic events in a chalcogenide memristor.Sci Rep. 2013;3:1619. doi: 10.1038/srep01619. Sci Rep. 2013. PMID: 23563810 Free PMC article.
-
The timing of activity is a regulatory signal during development of neural connections.J Mol Neurosci. 2014 Jul;53(3):324-9. doi: 10.1007/s12031-013-0128-z. Epub 2013 Oct 10. J Mol Neurosci. 2014. PMID: 24114571 Review.
-
Synaptic inputs compete during rapid formation of the calyx of Held: a new model system for neural development.J Neurosci. 2013 Aug 7;33(32):12954-69. doi: 10.1523/JNEUROSCI.1087-13.2013. J Neurosci. 2013. PMID: 23926251 Free PMC article.
-
Adult rat motor neurons do not re-establish electrical coupling during axonal regeneration and muscle reinnervation.PLoS One. 2015 Apr 13;10(4):e0123576. doi: 10.1371/journal.pone.0123576. eCollection 2015. PLoS One. 2015. PMID: 25875765 Free PMC article.
References
-
- Hubel DH, Wiesel TN. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965;28:1041–1059. - PubMed
-
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. - PubMed
-
- Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

