Spg20-/- mice reveal multimodal functions for Troyer syndrome protein spartin in lipid droplet maintenance, cytokinesis and BMP signaling
- PMID: 22619377
- PMCID: PMC3406757
- DOI: 10.1093/hmg/dds191
Spg20-/- mice reveal multimodal functions for Troyer syndrome protein spartin in lipid droplet maintenance, cytokinesis and BMP signaling
Abstract
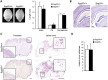
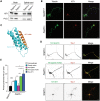
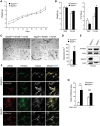
Hereditary spastic paraplegias (HSPs; SPG1-48) are inherited neurological disorders characterized by lower extremity spasticity and weakness. Loss-of-function mutations in the SPG20 gene encoding spartin cause autosomal recessive Troyer syndrome (SPG20), which has additional features of short stature, cognitive deficits and distal amyotrophy. To identify cellular impairments underlying Troyer syndrome, we generated Spg20-/- mice, which exhibit progressive gait defects. Although gross central nervous system pathology appeared largely normal, cerebral cortical neurons cultured from neonatal Spg20-/- mice exhibited increased axon branching, a phenotype suppressed by reintroducing spartin and which required its interaction with the endosomal sorting complex required for transport (ESCRT)-III protein IST1. Analysis of the bone morphogenetic protein (BMP) signaling pathway in Spg20-/- embryonic fibroblasts indicated that Smad1/5 phosphorylation is modestly elevated, possibly due to alterations in BMP receptor trafficking. Cytokinesis was impaired in embryonic fibroblasts cultured from Spg20-/- mice, and binucleated chondrocytes were prominent in epiphyseal growth plates of bones in Spg20-/- mice, perhaps explaining the short stature of patients. Finally, adipose tissue from Spg20-/- female mice exhibited increased lipid droplet (LD) numbers and alterations in perilipin levels, supporting a role for spartin in LD maintenance. Taken together, our results support multimodal functions for spartin that provide important insights into HSP pathogenesis.
Figures




Similar articles
-
SPG20 protein spartin is recruited to midbodies by ESCRT-III protein Ist1 and participates in cytokinesis.Mol Biol Cell. 2010 Oct 1;21(19):3293-303. doi: 10.1091/mbc.E09-10-0879. Epub 2010 Aug 18. Mol Biol Cell. 2010. PMID: 20719964 Free PMC article.
-
The Troyer syndrome (SPG20) protein spartin interacts with Eps15.Biochem Biophys Res Commun. 2005 Sep 9;334(4):1042-8. doi: 10.1016/j.bbrc.2005.06.201. Biochem Biophys Res Commun. 2005. PMID: 16036216
-
A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover.J Cell Biol. 2009 Mar 23;184(6):881-94. doi: 10.1083/jcb.200808041. J Cell Biol. 2009. PMID: 19307600 Free PMC article.
-
Dwarfism in Troyer syndrome: a family with SPG20 compound heterozygous mutations and a literature review.Ann N Y Acad Sci. 2020 Feb;1462(1):118-127. doi: 10.1111/nyas.14229. Epub 2019 Sep 19. Ann N Y Acad Sci. 2020. PMID: 31535723 Review.
-
Spartin: At the crossroad between ubiquitination and metabolism in cancer.Biochim Biophys Acta Rev Cancer. 2022 Nov;1877(6):188813. doi: 10.1016/j.bbcan.2022.188813. Epub 2022 Oct 1. Biochim Biophys Acta Rev Cancer. 2022. PMID: 36195276 Review.
Cited by
-
Overexpression of OLC1 promotes tumorigenesis of human esophageal squamous cell carcinoma.PLoS One. 2014 Mar 7;9(3):e90958. doi: 10.1371/journal.pone.0090958. eCollection 2014. PLoS One. 2014. PMID: 24608342 Free PMC article.
-
Impaired lipid metabolism in astrocytes underlies degeneration of cortical projection neurons in hereditary spastic paraplegia.Acta Neuropathol Commun. 2020 Dec 7;8(1):214. doi: 10.1186/s40478-020-01088-0. Acta Neuropathol Commun. 2020. PMID: 33287888 Free PMC article.
-
Strumpellin and Spartin, Hereditary Spastic Paraplegia Proteins, are Binding Partners.J Exp Neurosci. 2015 May 7;9:15-25. doi: 10.4137/JEN.S22969. eCollection 2015. J Exp Neurosci. 2015. PMID: 25987849 Free PMC article.
-
An Automated Imaging-Based Screen for Genetic Modulators of ER Organisation in Cultured Human Cells.Cells. 2024 Mar 26;13(7):577. doi: 10.3390/cells13070577. Cells. 2024. PMID: 38607016 Free PMC article.
-
Role of glial cells in motor neuron degeneration in hereditary spastic paraplegias.Front Cell Neurosci. 2025 Apr 15;19:1553658. doi: 10.3389/fncel.2025.1553658. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40302786 Free PMC article. Review.
References
-
- Salinas S., Proukakis C., Crosby A., Warner T.T. Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol. 2008;7:1127–1138. - PubMed
-
- Harding A.E. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1:1151–1155. - PubMed
-
- Fink J.K. Hereditary spastic paraplegia. Curr. Neurol. Neurosci. Rep. 2006;6:65–76. - PubMed
-
- Soderblom C., Blackstone C. Traffic accidents: molecular genetic insights into the pathogenesis of the hereditary spastic paraplegias. Pharmacol. Ther. 2006;109:42–56. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

