Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: a potential theranostic agent
- PMID: 22619538
- PMCID: PMC3356180
- DOI: 10.2147/IJN.S29242
Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: a potential theranostic agent
Abstract
Objectives: Drug delivery systems consisting of liposomes displaying a cell surface receptor-targeting peptide are being developed to specifically deliver chemotherapeutic drugs to tumors overexpressing a target receptor. This study addresses novel liposome composition approaches to specifically target tissues overexpressing bombesin (BN) receptors.
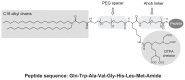
Methods: A new amphiphilic peptide derivative (MonY-BN) containing the BN(7-14) peptide, the DTPA (diethylenetriaminepentaacetate) chelating agent, a hydrophobic moiety with two C(18) alkyl chains, and polyethylene glycol spacers, has been synthesized by solid-phase methods. Liposomes have been generated by co-aggregation of MonY-BN with 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC). The structural and biological properties of these new target-selective drug-delivery systems have been characterized.
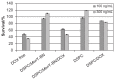
Results: Liposomes with a DSPC/MonY-BN (97/3 molar ratio) composition showed a diameter of 145.5 ± 31.5 nm and a polydispersity index of 0.20 ± 0.05. High doxorubicin (Dox) loading was obtained with the remote pH gradient method using citrate as the inner buffer. Specific binding to PC-3 cells of DSPC/MonY-BN liposomes was obtained (2.7% ± 0.3%, at 37°C), compared with peptide-free DSPC liposomes (1.4% ± 0.2% at 37°C). Incubation of cells with DSPC/ MonY-BN/Dox showed significantly lower cell survival compared with DSPC/Dox-treated cells, in the presence of 100 ng/mL and 300 ng/mL drug amounts, in cytotoxicity experiments. Intravenous treatment of PC-3 xenograft-bearing mice with DSPC/MonY-BN/Dox at 10 mg/kg Dox dose produced higher tumour growth inhibition (60%) compared with nonspecific DSPC/ Dox liposomes (36%) relative to control animals.
Conclusion: The structural and loading properties of DSPC/MonY-BN liposomes along with the observed in-vitro and in-vivo activity are encouraging for further development of this approach for target-specific cancer chemotherapy.
Keywords: PC-3 cells; bombesin peptide; doxorubicin delivery; gastrin-releasing peptide receptors; theranostic applications.
Figures


References
-
- Chabner BA, Allegra CJ, Curt GA, Calabresi P. Antineoplastic agents. In: Hardman JG, Limbird LE, editors. Goodman and Gillman’s The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw-Hill; 1996. p. 1265.
-
- Hortobagyi GN. Anthracyclines in the treatment of cancer: an overview. Drugs. 1997;54(Suppl 4):1–7. - PubMed
-
- Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin: rationale for use in solid tumours. Drugs. 1997;54(Suppl 4):15–21. - PubMed
-
- Gabizon A. Pharmacokinetics of PEGylated liposomal doxorubicin. Clin Pharmacokinet. 2003;42:419–436. - PubMed
-
- Abraham SA, Waterhouse DN, Lawrence D, et al. The liposomal formulation of doxorubicin. Methods Enzymol. 2005;391:71–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

