Myostatin is a novel tumoral factor that induces cancer cachexia
- PMID: 22621320
- PMCID: PMC3408049
- DOI: 10.1042/BJ20112024
Myostatin is a novel tumoral factor that induces cancer cachexia
Erratum in
-
1) Myostatin is a novel tumoral factor that induces cancer cachexia.Biochem J. 2015 Feb 15;466(1):201. doi: 10.1042/bj4660201u. Biochem J. 2015. PMID: 25656055 Free PMC article. No abstract available.
Retraction in
-
Retraction: Myostatin is a novel tumoral factor that induces cancer cachexia.Biochem J. 2016 Apr 15;473(8):1111. doi: 10.1042/BJ4731111. Biochem J. 2016. PMID: 27060107 Free PMC article. No abstract available.
Abstract
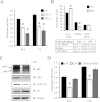
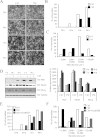
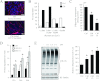
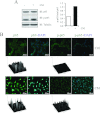
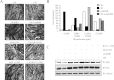
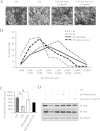
Humoral and tumoral factors collectively promote cancer-induced skeletal muscle wasting by increasing protein degradation. Although several humoral proteins, namely TNFα (tumour necrosis factor α) and IL (interleukin)-6, have been shown to induce skeletal muscle wasting, there is a lack of information regarding the tumoral factors that contribute to the atrophy of muscle during cancer cachexia. Therefore, in the present study, we have characterized the secretome of C26 colon cancer cells to identify the tumoral factors involved in cancer-induced skeletal muscle wasting. In the present study, we show that myostatin, a procachectic TGFβ (transforming growth factor β) superfamily member, is abundantly secreted by C26 cells. Consistent with myostatin signalling during cachexia, treating differentiated C2C12 myotubes with C26 CM (conditioned medium) resulted in myotubular atrophy due to the up-regulation of muscle-specific E3 ligases, atrogin-1 and MuRF1 (muscle RING-finger protein 1), and enhanced activity of the ubiquitin-proteasome pathway. Furthermore, the C26 CM also activated ActRIIB (activin receptor type II B)/Smad and NF-κB (nuclear factor κB) signalling, and reduced the activity of the IGF-I (insulin-like growth factor 1)/PI3K (phosphoinositide 3-kinase)/Akt pathway, three salient molecular features of myostatin action in skeletal muscles. Antagonists to myostatin prevented C26 CM-induced wasting in muscle cell cultures, further confirming that tumoral myostatin may be a key contributor in the pathogenesis of cancer cachexia. Finally, we show that treatment with C26 CM induced the autophagy-lysosome pathway and reduced the number of mitochondria in myotubes. These two previously unreported observations were recapitulated in skeletal muscles collected from C26 tumour-bearing mice.
Figures



References
-
- Tisdale M. J. Mechanisms of cancer cachexia. Physiol. Rev. 2009;89:381–410. - PubMed
-
- Tisdale M. J. Molecular pathways leading to cancer cachexia. Physiology. 2005;20:340–348. - PubMed
-
- Tisdale M. J. The ubiquitin-proteasome pathway as a therapeutic target for muscle wasting. J. Support. Oncol. 2005;3:209–217. - PubMed
-
- Fearon K. C. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur. J. Cancer. 2008;44:1124–1132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

