Structural genomics plucks high-hanging membrane proteins
- PMID: 22622032
- PMCID: PMC3400333
- DOI: 10.1016/j.sbi.2012.05.002
Structural genomics plucks high-hanging membrane proteins
Abstract
Recent years have seen the establishment of structural genomics centers that explicitly target integral membrane proteins. Here, we review the advances in targeting these extremely high-hanging fruits of structural biology in high-throughput mode. We observe that the experimental determination of high-resolution structures of integral membrane proteins is increasingly successful both in terms of getting structures and of covering important protein families, for example, from Pfam. Structural genomics has begun to contribute significantly toward this progress. An important component of this contribution is the set up of robotic pipelines that generate a wealth of experimental data for membrane proteins. We argue that prediction methods for the identification of membrane regions and for the comparison of membrane proteins largely suffice to meet the challenges of target selection for structural genomics of membrane proteins. In contrast, we need better methods to prioritize the most promising members in a family of closely related proteins and to annotate protein function from sequence and structure in absence of homology.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
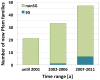

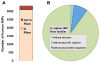

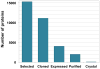
References
-
- von Heijne G. The membrane protein universe: what’s out there and why bother? J Intern Med. 2007;261:543–557. - PubMed
-
- von Heijne G. Membrane-protein topology. Nat Rev Mol Cell Biol. 2006;7:909–918. - PubMed
-
- Fagerberg L, Jonasson K, von Heijne G, Uhlen M, Berglund L. Prediction of the human membrane proteome. Proteomics. 2010;10:1141–1149. - PubMed
-
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

