mTORC1 is essential for leukemia propagation but not stem cell self-renewal
- PMID: 22622041
- PMCID: PMC3366413
- DOI: 10.1172/JCI62279
mTORC1 is essential for leukemia propagation but not stem cell self-renewal
Abstract
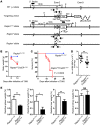
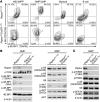
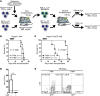
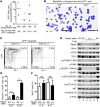
Although dysregulation of mTOR complex 1 (mTORC1) promotes leukemogenesis, how mTORC1 affects established leukemia is unclear. We investigated the role of mTORC1 in mouse hematopoiesis using a mouse model of conditional deletion of Raptor, an essential component of mTORC1. Raptor deficiency impaired granulocyte and B cell development but did not alter survival or proliferation of hematopoietic progenitor cells. In a mouse model of acute myeloid leukemia (AML), Raptor deficiency significantly suppressed leukemia progression by causing apoptosis of differentiated, but not undifferentiated, leukemia cells. mTORC1 did not control cell cycle or cell growth in undifferentiated AML cells in vivo. Transplantation of Raptor-deficient undifferentiated AML cells in a limiting dilution revealed that mTORC1 is essential for leukemia initiation. Strikingly, a subset of AML cells with undifferentiated phenotypes survived long-term in the absence of mTORC1 activity. We further demonstrated that the reactivation of mTORC1 in those cells restored their leukemia-initiating capacity. Thus, AML cells lacking mTORC1 activity can self-renew as AML stem cells. Our findings provide mechanistic insight into how residual tumor cells circumvent anticancer therapies and drive tumor recurrence.
Figures





Comment in
-
Getting to the finish line with mTORC1-targeted therapy.J Clin Invest. 2012 Jun;122(6):1970-2. doi: 10.1172/JCI64227. Epub 2012 May 24. J Clin Invest. 2012. PMID: 22622033 Free PMC article.
References
-
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

