In vitro differentiation of mouse embryonic stem cells into inner ear hair cell-like cells using stromal cell conditioned medium
- PMID: 22622133
- PMCID: PMC3366087
- DOI: 10.1038/cddis.2012.56
In vitro differentiation of mouse embryonic stem cells into inner ear hair cell-like cells using stromal cell conditioned medium
Abstract
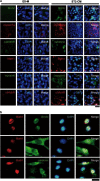
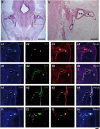
Hearing loss is mainly caused by loss of sensory hair cells (HCs) in the organ of Corti or cochlea. Although embryonic stem (ES) cells are a promising source for cell therapy, little is known about the efficient generation of HC-like cells from ES cells. In the present study, we developed a single-medium culture method for growing embryoid bodies (EBs), in which conditioned medium (CM) from cultures of ST2 stromal cells (ST2-CM) was used for 14-day cultures of 4-day EBs. At the end of the 14-day cultures, up to 20% of the cells in EB outgrowths expressed HC-related markers, including Math1 (also known as Atoh1), myosin6, myosin7a, calretinin, α9AchR and Brn3c (also known as Pou4f3), and also showed formation of stereocilia-like structures. Further, we found that these cells were incorporated into the developing inner ear after transplantation into chick embryos. The present inner ear HC induction method using ST2-CM (HIST2 method) is quite simple and highly efficient to obtain ES-derived HC-like cells with a relatively short cultivation time.
Figures




References
-
- Carey J, Amin N. Evolutionary changes in the cochlea and labyrinth: Solving the problem of sound transmission to the balance organs of the inner ear. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:482–489. - PubMed
-
- Raphael Y. Cochlear pathology, sensory cell death and regeneration. Br Med Bull. 2002;63:25–38. - PubMed
-
- Fekete DM. Cell fate specification in the inner ear. Curr Opin Neurobiol. 1996;6:533–541. - PubMed
-
- Holley MC. Application of new biological approaches to stimulate sensory repair and protection. Br Med Bull. 2002;63:157–169. - PubMed
-
- Hu Z, Wei D, Johansson CB, Holmström N, Duan M, Frisén J, et al. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005;302:40–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

