Screening a cDNA library for protein-protein interactions directly in planta
- PMID: 22623495
- PMCID: PMC3442567
- DOI: 10.1105/tpc.112.097998
Screening a cDNA library for protein-protein interactions directly in planta
Abstract
Screening cDNA libraries for genes encoding proteins that interact with a bait protein is usually performed in yeast. However, subcellular compartmentation and protein modification may differ in yeast and plant cells, resulting in misidentification of protein partners. We used bimolecular fluorescence complementation technology to screen a plant cDNA library against a bait protein directly in plants. As proof of concept, we used the N-terminal fragment of yellow fluorescent protein- or nVenus-tagged Agrobacterium tumefaciens VirE2 and VirD2 proteins and the C-terminal extension (CTE) domain of Arabidopsis thaliana telomerase reverse transcriptase as baits to screen an Arabidopsis cDNA library encoding proteins tagged with the C-terminal fragment of yellow fluorescent protein. A library of colonies representing ~2 × 10(5) cDNAs was arrayed in 384-well plates. DNA was isolated from pools of 10 plates, individual plates, and individual rows and columns of the plates. Sequential screening of subsets of cDNAs in Arabidopsis leaf or tobacco (Nicotiana tabacum) Bright Yellow-2 protoplasts identified single cDNA clones encoding proteins that interact with either, or both, of the Agrobacterium bait proteins, or with CTE. T-DNA insertions in the genes represented by some cDNAs revealed five novel Arabidopsis proteins important for Agrobacterium-mediated plant transformation. We also used this cDNA library to confirm VirE2-interacting proteins in orchid (Phalaenopsis amabilis) flowers. Thus, this technology can be applied to several plant species.
Figures



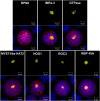

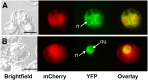




References
-
- Bartel P.L., Fields S. (1997). The Yeast Two-Hybrid System. (New York: Oxford University Press: ).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

