Some strains of Plasmodium falciparum, a human malaria parasite, evade the complement-like system of Anopheles gambiae mosquitoes
- PMID: 22623529
- PMCID: PMC3396512
- DOI: 10.1073/pnas.1121183109
Some strains of Plasmodium falciparum, a human malaria parasite, evade the complement-like system of Anopheles gambiae mosquitoes
Abstract
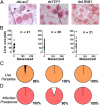
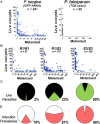
Plasmodium falciparum lines differ in their ability to infect mosquitoes. The Anopheles gambiae L3-5 refractory (R) line melanizes most Plasmodium species, including the Brazilian P. falciparum 7G8 line, but it is highly susceptible to some African P. falciparum strains such as 3D7, NF54, and GB4. We investigated whether these lines differ in their ability to evade the mosquito immune system. Silencing key components of the mosquito complement-like system [thioester-containing protein 1 (TEP1), leucine-rich repeat protein 1, and Anopheles Plasmodium-responsive leucine-rich repeat protein 1] prevented melanization of 7G8 parasites, reverting the refractory phenotype. In contrast, it had no effect on the intensity of infection with NF54, suggesting that this line is able to evade TEP1-mediated lysis. When R females were coinfected with a line that is melanized (7G8) and a line that survives (3D7), the coinfection resulted in mixed infections with both live and encapsulated parasites on individual midguts. This finding shows that survival of individual parasites is parasite-specific and not systemic in nature, because parasites can evade TEP1-mediated lysis even when other parasites are melanized in the same midgut. When females from an extensive genetic cross between R and susceptible A. gambiae (G3) mosquitoes were infected with P. berghei, encapsulation was strongly correlated with the TEP1-R1 allele. However, P. falciparum 7G8 parasites were no longer encapsulated by females from this cross, indicating that the TEP1-R1 allele is not sufficient to melanize this line. Evasion of the A. gambiae immune system by P. falciparum may be the result of parasite adaptation to sympatric mosquito vectors and may be an important factor driving malaria transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Health Organization W . World Malaria Report. Geneva: World Health Organization; 2010.
-
- Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: Metamorphoses of malaria in the mosquito. Trends Parasitol. 2005;21:573–580. - PubMed
-
- Vogel G. The ‘do unto others’ malaria vaccine. Science. 2010;328:847–848. - PubMed
-
- Collins FH, et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

