Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts
- PMID: 22623533
- PMCID: PMC3386073
- DOI: 10.1073/pnas.1121606109
Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts
Abstract
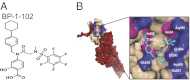
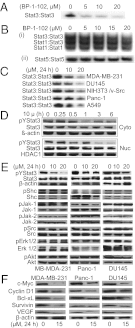
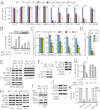
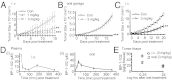
Computer-aided lead optimization derives a unique, orally bioavailable inhibitor of the signal transducer and activator of transcription (Stat)3 Src homology 2 domain. BP-1-102 binds Stat3 with an affinity (K(D)) of 504 nM, blocks Stat3-phospho-tyrosine (pTyr) peptide interactions and Stat3 activation at 4-6.8 μM, and selectively inhibits growth, survival, migration, and invasion of Stat3-dependent tumor cells. BP-1-102-mediated inhibition of aberrantly active Stat3 in tumor cells suppresses the expression of c-Myc, Cyclin D1, Bcl-xL, Survivin, VEGF, and Krüppel-like factor 8, which is identified as a Stat3 target gene that promotes Stat3-mediated breast tumor cell migration and invasion. Treatment of breast cancer cells with BP-1-102 further blocks Stat3-NF-κB cross-talk, the release of granulocyte colony-stimulating factor, soluble intercellular adhesion molecule 1, macrophage migration-inhibitory factor/glycosylation-inhibiting factor, interleukin 1 receptor antagonist, and serine protease inhibitor protein 1, and the phosphorylation of focal adhesion kinase and paxillin, while enhancing E-cadherin expression. Intravenous or oral gavage delivery of BP-1-102 furnishes micromolar or microgram levels in tumor tissues and inhibits growth of human breast and lung tumor xenografts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. - PubMed
-
- Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. - PubMed
-
- Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409–422. - PubMed
-
- Wang T, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10(1):48–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

