Fibroblast growth factor 8 organizes the neocortical area map and regulates sensory map topography
- PMID: 22623663
- PMCID: PMC3466079
- DOI: 10.1523/JNEUROSCI.0071-12.2012
Fibroblast growth factor 8 organizes the neocortical area map and regulates sensory map topography
Abstract
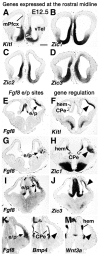
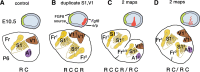
The concept of an "organizer" is basic to embryology. An organizer is a portion of the embryo producing signals that lead to the creation of a patterned mature structure from an embryonic primordium. Fibroblast growth factor 8 (FGF8) is a morphogen that disperses from a rostromedial source in the neocortical primordium (NP), forms a rostral-to-caudal (R/C) gradient, and regulates embryonic and neonatal R/C patterns of gene expression in neocortex. Whether FGF8 also has organizer activity that generates the postnatal neocortical area map is uncertain. To test this possibility, new sources of FGF8 were introduced into the mouse NP with in utero microelectroporation at embryonic day 10.5, close to the estimated peak of area patterning. Results differed depending on the position of ectopic FGF8. Ectopic FGF8 in the caudalmost NP could duplicate somatosensory cortex (S1) and primary visual cortex (V1). FGF8 delivered to the midlateral NP generated a sulcus separating rostral and caudal portions of the NP, in effect creating duplicate NPs. In the caudal NP, ectopic FGF8 induced a second, inclusive area map, containing frontal cortex, S1, V1, and primary auditory areas. Moreover, duplicate S1 showed plasticity to sensory deprivation, and duplicate V1 responded to visual stimuli. Our findings implicate FGF8 as an organizer signal, and its source in the rostromedial telencephalon as an organizer of the neocortical area map.
Figures









References
-
- Agarwala S, Sanders TA, Ragsdale CW. Sonic hedgehog control of size and shape in midbrain pattern formation. Science. 2001;291:2147–2150. - PubMed
-
- Armentano M, Chou SJ, Tomassy GS, Leingärtner A, O'Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. - PubMed
-
- Aruga J. The role of Zic genes in neural development. Mol Cell Neurosci. 2004;26:205–221. - PubMed
-
- Bishop KM, Goudreau G, O'Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
