PTH/PTHrP and vitamin D control antimicrobial peptide expression and susceptibility to bacterial skin infection
- PMID: 22623742
- PMCID: PMC3725981
- DOI: 10.1126/scitranslmed.3003759
PTH/PTHrP and vitamin D control antimicrobial peptide expression and susceptibility to bacterial skin infection
Abstract
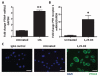
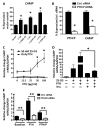
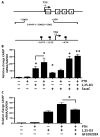
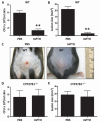
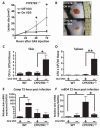
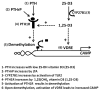
The production of antimicrobial peptides is essential for protection against a wide variety of microbial pathogens and plays an important role in the pathogenesis of several diseases. The mechanisms responsible for expression of antimicrobial peptides are incompletely understood, but a role for vitamin D as a transcriptional inducer of the antimicrobial peptide cathelicidin has been proposed. We show that 1,25-dihydroxyvitamin D(3) (1,25-D3) acts together with parathyroid hormone (PTH), or the shared amino-terminal domain of PTH-related peptide (PTHrP), to synergistically increase cathelicidin and immune defense. Administration of PTH to mouse skin decreased susceptibility to skin infection by group A Streptococcus. Mice on dietary vitamin D(3) restriction that responded with an elevation in PTH have an increased risk of infection if they lack 1,25-D3. These results identify PTH/PTHrP as a variable that serves to compensate for inadequate vitamin D during activation of antimicrobial peptide production.
Figures
Similar articles
-
Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin.J Immunol. 2008 Jun 1;180(11):7565-73. doi: 10.4049/jimmunol.180.11.7565. J Immunol. 2008. PMID: 18490758 Free PMC article.
-
Vitamin D regulation of cathelicidin in the skin: toward a renaissance of vitamin D in dermatology?J Invest Dermatol. 2008 Apr;128(4):773-5. doi: 10.1038/jid.2008.35. J Invest Dermatol. 2008. PMID: 18337709
-
Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection.Proc Natl Acad Sci U S A. 2005 Mar 8;102(10):3750-5. doi: 10.1073/pnas.0500268102. Epub 2005 Feb 22. Proc Natl Acad Sci U S A. 2005. PMID: 15728389 Free PMC article.
-
Recent studies on the biological action of parathyroid hormone (PTH)-related peptide (PTHrP) and PTH/PTHrP receptor in cartilage and bone.Histol Histopathol. 2000 Jul;15(3):957-70. doi: 10.14670/HH-15.957. Histol Histopathol. 2000. PMID: 10963138 Review.
-
[The PTH/PTHrP receptor: biological implications].Nefrologia. 2003;23 Suppl 2:12-7. Nefrologia. 2003. PMID: 12778847 Review. Spanish.
Cited by
-
Bone marrow-induced Mef2c deficiency delays B-cell development and alters the expression of key B-cell regulatory proteins.Int Immunol. 2013 Feb;25(2):99-115. doi: 10.1093/intimm/dxs088. Epub 2012 Oct 19. Int Immunol. 2013. PMID: 23087187 Free PMC article.
-
Immunoregulation in cancer-associated cachexia.J Adv Res. 2024 Apr;58:45-62. doi: 10.1016/j.jare.2023.04.018. Epub 2023 May 6. J Adv Res. 2024. PMID: 37150253 Free PMC article. Review.
-
Vitamin D and skin disorders: bridging molecular insights to clinical innovations.Mol Med. 2025 Jul 18;31(1):259. doi: 10.1186/s10020-025-01311-5. Mol Med. 2025. PMID: 40681996 Free PMC article. Review.
-
Protective actions of vitamin D in UVB induced skin cancer.Photochem Photobiol Sci. 2012 Dec;11(12):1808-16. doi: 10.1039/c2pp25251a. Photochem Photobiol Sci. 2012. PMID: 22990497 Free PMC article. Review.
-
New aspects of vitamin D metabolism and action - addressing the skin as source and target.Nat Rev Endocrinol. 2020 Apr;16(4):234-252. doi: 10.1038/s41574-019-0312-5. Epub 2020 Feb 6. Nat Rev Endocrinol. 2020. PMID: 32029884 Review.
References
-
- Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J. Invest. Dermatol. 2005;125:9–13. - PubMed
-
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002;347:1151–1160. - PubMed
-
- Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zügel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D–dependent mechanism. J. Clin. Invest. 2007;117:803–811. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

