An Epstein-Barr Virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model
- PMID: 22623780
- PMCID: PMC3421695
- DOI: 10.1128/JVI.00770-12
An Epstein-Barr Virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model
Abstract
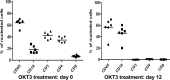
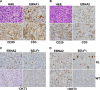
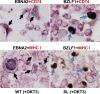
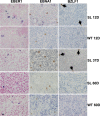
Immunosuppressed patients are at risk for developing Epstein-Barr Virus (EBV)-positive lymphomas that express the major EBV oncoprotein, LMP1. Although increasing evidence suggests that a small number of lytically infected cells may promote EBV-positive lymphomas, the impact of enhanced lytic gene expression on the ability of EBV to induce lymphomas is unclear. Here we have used immune-deficient mice, engrafted with human fetal hematopoietic stem cells and thymus and liver tissue, to compare lymphoma formation following infection with wild-type (WT) EBV versus infection with a "superlytic" (SL) mutant with enhanced BZLF1 (Z) expression. The same proportions (2/6) of the WT and SL virus-infected animals developed B-cell lymphomas by day 60 postinfection; the remainder of the animals had persistent tumor-free viral latency. In contrast, all WT and SL virus-infected animals treated with the OKT3 anti-CD3 antibody (which inhibits T-cell function) developed lymphomas by day 29. Lymphomas in OKT3-treated animals (in contrast to lymphomas in the untreated animals) contained many LMP1-expressing cells. The SL virus-infected lymphomas in both OKT3-treated and untreated animals contained many more Z-expressing cells (up to 30%) than the WT virus-infected lymphomas, but did not express late viral proteins and thus had an abortive lytic form of EBV infection. LMP1 and BMRF1 (an early lytic viral protein) were never coexpressed in the same cell, suggesting that LMP1 expression is incompatible with lytic viral reactivation. These results show that the SL mutant induces an "abortive" lytic infection in humanized mice that is compatible with continued cell growth and at least partially resistant to T-cell killing.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

