Suppression of the interferon and NF-κB responses by severe fever with thrombocytopenia syndrome virus
- PMID: 22623799
- PMCID: PMC3421730
- DOI: 10.1128/JVI.00612-12
Suppression of the interferon and NF-κB responses by severe fever with thrombocytopenia syndrome virus
Abstract
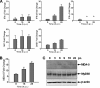
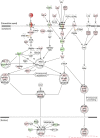
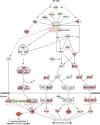
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease characterized by high fever, thrombocytopenia, multiorgan dysfunction, and a high fatality rate between 12 and 30%. It is caused by SFTS virus (SFTSV), a novel Phlebovirus in family Bunyaviridae. Although the viral pathogenesis remains largely unknown, hemopoietic cells appear to be targeted by the virus. In this study we report that human monocytes were susceptible to SFTSV, which replicated efficiently, as shown by an immunofluorescence assay and real-time reverse transcription-PCR. We examined host responses in the infected cells and found that antiviral interferon (IFN) and IFN-inducible proteins were induced upon infection. However, our data also indicated that downregulation of key molecules such as mitochondrial antiviral signaling protein (MAVS) or weakened activation of interferon regulatory factor (IRF) and NF-κB responses may contribute to a restricted innate immunity against the infection. NSs, the nonstructural protein encoded by the S segment, suppressed the beta interferon (IFN-β) and NF-κB promoter activities, although NF-κB activation appears to facilitate SFTSV replication in human monocytes. NSs was found to be associated with TBK1 and may inhibit the activation of downstream IRF and NF-κB signaling through this interaction. Interestingly, we demonstrated that the nucleoprotein (N), also encoded by the S segment, exhibited a suppressive effect on the activation of IFN-β and NF-κB signaling as well. Infected monocytes, mainly intact and free of apoptosis, may likely be implicated in persistent viral infection, spreading the virus to the circulation and causing primary viremia. Our findings provide the first evidence in dissecting the host responses in monocytes and understanding viral pathogenesis in humans infected with a novel deadly Bunyavirus.
Figures






References
-
- Bachmann M, Moroy T. 2005. The serine/threonine kinase Pim-1. Int. J. Biochem. Cell Biol. 37:726–730 - PubMed
-
- Bao CJ, et al. 2007. A family cluster of infections by a newly recognized bunyavirus in eastern China: further evidence of person-to-person transmission. Clin. Infect. Dis. 53:1208–1214 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

