Monocytes do not transdifferentiate into proper osteoblasts
- PMID: 22623892
- PMCID: PMC3353470
- DOI: 10.1100/2012/384936
Monocytes do not transdifferentiate into proper osteoblasts
Abstract
Recent publications suggested that monocytes might be an attractive cell type to transdifferentiate into various cellular phenotypes. Aim was, therefore, to evaluate the potential of blood monocytes to transdifferentiate into osteoblasts. Monocytes isolated from peripheral blood were subjected to two previously published treatments to obtain unique, multipotent cell fractions, named programmable cells of monocytic origin (PCMOs) and monocyte-derived mesenchymal progenitor cells (MOMPs). Subsequently, MOMPs and PCMOs were treated with osteogenic differentiation medium (including either vitamin D or dexamethasone) for 14 days. Regarding a variety of surface markers, no differences between MOMPs, PCMOs, and primary monocytes could be detected. The treatment with osteogenic medium neither resulted in loss of hematopoietic markers nor in adoption of mesenchymal phenotype in all cell types. No significant effect was observed regarding the expression of osteogenic transcription factors, bone-related genes, or production of mineralized matrix. Osteogenic medium resulted in activation of monocytes and appearance of osteoclasts. In conclusion, none of the investigated monocyte cell types showed any transdifferentiation characteristics under the tested circumstances. Based on our data, we rather see an activation and maturation of monocytes towards macrophages and osteoclasts.
Figures

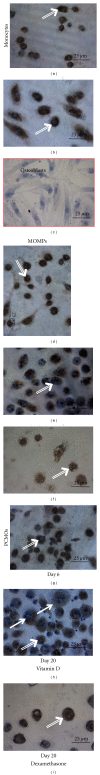
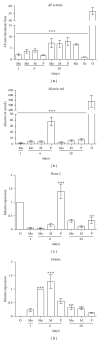


References
-
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. - PubMed
-
- Bain BJ. Bone marrow biopsy morbidity and mortality. British Journal of Haematology. 2003;121(6):949–951. - PubMed
-
- Ehnert S, Seeliger C, Vester H, et al. Autologous serum improves yield and metabolic capacity of monocyte-derived hepatocyte-like cells: possible implication for cell transplantation. Cell Transplantation. 2011;20(9):1465–1477. - PubMed
-
- Seta N, Kuwana M. Human circulating monocytes as multipotential progenitors. Keio Journal of Medicine. 2007;56(2):41–47. - PubMed
-
- Zhao XY, Li W, Lv Z, et al. IPS cells produce viable mice through tetraploid complementation. Nature. 2009;461(7260):86–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

