Global microRNA expression profiling of high-risk ER+ breast cancers from patients receiving adjuvant tamoxifen mono-therapy: a DBCG study
- PMID: 22623953
- PMCID: PMC3356496
- DOI: 10.1371/journal.pone.0036170
Global microRNA expression profiling of high-risk ER+ breast cancers from patients receiving adjuvant tamoxifen mono-therapy: a DBCG study
Abstract
Purpose: Despite the benefits of estrogen receptor (ER)-targeted endocrine therapies in breast cancer, many tumors develop resistance. MicroRNAs (miRNAs) have been suggested as promising biomarkers and we here evaluated whether a miRNA profile could be identified, sub-grouping ER+ breast cancer patients treated with adjuvant Tamoxifen with regards to probability of recurrence.
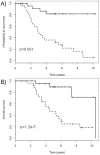
Experimental design: Global miRNA analysis was performed on 152 ER+ primary tumors from high-risk breast cancer patients with an initial discovery set of 52 patients, followed by two independent test sets (N = 60 and N = 40). All patients had received adjuvant Tamoxifen as mono-therapy (median clinical follow-up: 4.6 years) and half had developed distant recurrence (median time-to-recurrence: 3.5 years). MiRNA expression was examined by unsupervised hierarchical clustering and supervised analysis, including clinical parameters as co-variables.
Results: The discovery set identified 10 highly significant miRNAs that discriminated between the patient samples according to outcome. However, the subsequent two independent test sets did not confirm the predictive potential of these miRNAs. A significant correlation was identified between miR-7 and the tumor grade. Investigation of the microRNAs with the most variable expression between patients in different runs yielded a list of 31 microRNAs, eight of which are associated with stem cell characteristics.
Conclusions: Based on the large sample size, our data strongly suggests that there is no single miRNA profile predictive of outcome following adjuvant Tamoxifen treatment in a broad cohort of ER+ breast cancer patients. We identified a sub-group of Tamoxifen-treated breast cancer patients with miRNA-expressing tumors associated with cancer stem cell characteristics.
Conflict of interest statement
Figures


References
-
- Blamey RW. Guidelines on endocrine therapy of breast cancer EUSOMA. Eur J Cancer. 2002;38:615–634. - PubMed
-
- Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. - PubMed
-
- Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. - PubMed
-
- Mouridsen HT, Giobbie-Hurder A, Mauriac L, Paridaens R, Colleoni M, et al. BIG 1–98: A randomized double-blind phase III study evaluating letrozole and tamoxifen given in sequence as adjuvant endocrine therapy for postmenopausal women with receptor-positive breast cancer. SABCS, abstract 13: abstracts2view.com/sabcs/view.php? nu = SABCS08L_. 2008;553 BIG I–98 Collaborative, International Breast Cancer Study Group Bern .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

