A scalable system for production of functional pancreatic progenitors from human embryonic stem cells
- PMID: 22623968
- PMCID: PMC3356395
- DOI: 10.1371/journal.pone.0037004
A scalable system for production of functional pancreatic progenitors from human embryonic stem cells
Abstract
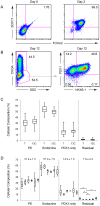
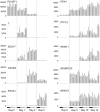
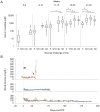
Development of a human embryonic stem cell (hESC)-based therapy for type 1 diabetes will require the translation of proof-of-principle concepts into a scalable, controlled, and regulated cell manufacturing process. We have previously demonstrated that hESC can be directed to differentiate into pancreatic progenitors that mature into functional glucose-responsive, insulin-secreting cells in vivo. In this study we describe hESC expansion and banking methods and a suspension-based differentiation system, which together underpin an integrated scalable manufacturing process for producing pancreatic progenitors. This system has been optimized for the CyT49 cell line. Accordingly, qualified large-scale single-cell master and working cGMP cell banks of CyT49 have been generated to provide a virtually unlimited starting resource for manufacturing. Upon thaw from these banks, we expanded CyT49 for two weeks in an adherent culture format that achieves 50-100 fold expansion per week. Undifferentiated CyT49 were then aggregated into clusters in dynamic rotational suspension culture, followed by differentiation en masse for two weeks with a four-stage protocol. Numerous scaled differentiation runs generated reproducible and defined population compositions highly enriched for pancreatic cell lineages, as shown by examining mRNA expression at each stage of differentiation and flow cytometry of the final population. Islet-like tissue containing glucose-responsive, insulin-secreting cells was generated upon implantation into mice. By four- to five-months post-engraftment, mature neo-pancreatic tissue was sufficient to protect against streptozotocin (STZ)-induced hyperglycemia. In summary, we have developed a tractable manufacturing process for the generation of functional pancreatic progenitors from hESC on a scale amenable to clinical entry.
Conflict of interest statement
Figures



References
-
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. - PubMed
-
- McCall MD, Toso C, Baetge EE, Shapiro AM. Are stem cells a cure for diabetes? Clin Sci (Lond) 2009;118:87–97. - PubMed
-
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

