Roles of molecular layer interneurons in sensory information processing in mouse cerebellar cortex Crus II in vivo
- PMID: 22623975
- PMCID: PMC3356402
- DOI: 10.1371/journal.pone.0037031
Roles of molecular layer interneurons in sensory information processing in mouse cerebellar cortex Crus II in vivo
Abstract
Background: Cerebellar cortical molecular layer interneurons (MLIs) play essential roles in sensory information processing by the cerebellar cortex. However, recent experimental and modeling results are questioning traditional roles for molecular layer inhibition in the cerebellum.
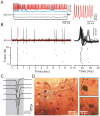
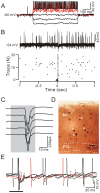
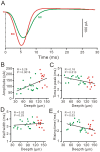
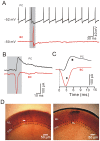
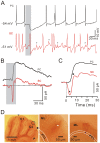
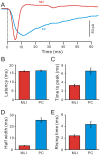
Methods and main results: Synaptic responses of MLIs and Purkinje cells (PCs), evoked by air-puff stimulation of the ipsilateral whisker pad were recorded from cerebellar cortex Crus II in urethane-anesthetized ICR mice by in vivo whole-cell patch-clamp recording techniques. Under current-clamp (I = 0), air-puff stimuli were found to primarily produce inhibition in PCs. In MLIs, this stimulus evoked spike firing regardless of whether they made basket-type synaptic connections or not. However, MLIs not making basket-type synaptic connections had higher rates of background activity and also generated spontaneous spike-lets. Under voltage-clamp conditions, excitatory postsynaptic currents (EPSCs) were recorded in MLIs, although the predominant response of recorded PCs was an inhibitory postsynaptic potential (IPSP). The latencies of EPSCs were similar for all MLIs, but the time course and amplitude of EPSCs varied with depth in the molecular layer. The highest amplitude, shortest duration EPSCs were recorded from MLIs deep in the molecular layer, which also made basket-type synaptic connections. Comparing MLI to PC responses, time to peak of PC IPSP was significantly slower than MLI recorded EPSCs. Blocking GABA(A) receptors uncovered larger EPSCs in PCs whose time to peak, half-width and 10-90% rising time were also significantly slower than in MLIs. Biocytin labeling indicated that the MLIs (but not PCs) are dye-coupled.
Conclusions: These findings indicate that tactile face stimulation evokes rapid excitation in MLIs and inhibition occurring at later latencies in PCs in mouse cerebellar cortex Crus II. These results support previous suggestions that the lack of parallel fiber driven PC activity is due to the effect of MLI inhibition.
Conflict of interest statement
Figures
Similar articles
-
Synaptic responses evoked by tactile stimuli in Purkinje cells in mouse cerebellar cortex Crus II in vivo.PLoS One. 2011;6(7):e22752. doi: 10.1371/journal.pone.0022752. Epub 2011 Jul 26. PLoS One. 2011. PMID: 21818384 Free PMC article.
-
Sensory stimulus evokes inhibition rather than excitation in cerebellar Purkinje cells in vivo in mice.Neurosci Lett. 2011 Jan 7;487(2):182-6. doi: 10.1016/j.neulet.2010.10.018. Epub 2010 Oct 19. Neurosci Lett. 2011. PMID: 20965231
-
Chronic ethanol exposure facilitates facial-evoked MLI-PC synaptic transmission via nitric oxide signaling pathway in vivo in mice.Neurosci Lett. 2020 Jan 10;715:134628. doi: 10.1016/j.neulet.2019.134628. Epub 2019 Nov 16. Neurosci Lett. 2020. PMID: 31738951
-
[Role of glutamate transporters in excitatory synapses in cerebellar Purkinje cells].Brain Nerve. 2007 Jul;59(7):669-76. Brain Nerve. 2007. PMID: 17663137 Review. Japanese.
-
Molecular Layer Interneurons: Key Elements of Cerebellar Network Computation and Behavior.Neuroscience. 2021 May 10;462:22-35. doi: 10.1016/j.neuroscience.2020.10.008. Epub 2020 Oct 17. Neuroscience. 2021. PMID: 33075461 Review.
Cited by
-
Electrical Synapses Enhance and Accelerate Interneuron Recruitment in Response to Coincident and Sequential Excitation.Front Cell Neurosci. 2018 Jun 19;12:156. doi: 10.3389/fncel.2018.00156. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29973871 Free PMC article.
-
Parallel fiber and climbing fiber responses in rat cerebellar cortical neurons in vivo.Front Syst Neurosci. 2013 May 17;7:16. doi: 10.3389/fnsys.2013.00016. eCollection 2013. Front Syst Neurosci. 2013. PMID: 23730272 Free PMC article.
-
Chronic Ethanol Consumption Impairs the Tactile-Evoked Long-Term Depression at Cerebellar Molecular Layer Interneuron-Purkinje Cell Synapses in vivo in Mice.Front Cell Neurosci. 2019 Jan 14;12:521. doi: 10.3389/fncel.2018.00521. eCollection 2018. Front Cell Neurosci. 2019. PMID: 30692916 Free PMC article.
-
Reconstruction and Simulation of a Scaffold Model of the Cerebellar Network.Front Neuroinform. 2019 May 15;13:37. doi: 10.3389/fninf.2019.00037. eCollection 2019. Front Neuroinform. 2019. PMID: 31156416 Free PMC article.
-
Corticotrophin-Releasing Factor Modulates the Facial Stimulation-Evoked Molecular Layer Interneuron-Purkinje Cell Synaptic Transmission in vivo in Mice.Front Cell Neurosci. 2020 Nov 26;14:563428. doi: 10.3389/fncel.2020.563428. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33324165 Free PMC article.
References
-
- Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine., Berlin: Springer-Verlag. 1967.
-
- Meek J. Why run parallel fibers parallel? Teleostean PCs as possible coincidence detectors, in a timing device subserving spatial coding of temporal differences. Neuroscience. 1992;48:249–283. - PubMed
-
- Eccles JC, Sabah NH, Schmidt RF, Taborikova H. Cutaneous mechanoreceptors influencing impulse discharges in cerebellar cortex. II. In Purkyn cells by mossy fiber input. Exp Brain Res. 1972;15:261–277. - PubMed
-
- Bower JM, Woolston DC. Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J Neurophysiol 49, 1983;745–766 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

