Superresolution imaging of ribosomes and RNA polymerase in live Escherichia coli cells
- PMID: 22624875
- PMCID: PMC3383343
- DOI: 10.1111/j.1365-2958.2012.08081.x
Superresolution imaging of ribosomes and RNA polymerase in live Escherichia coli cells
Abstract
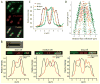
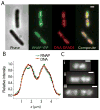
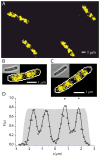
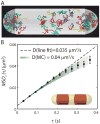
Quantitative spatial distributions of ribosomes (S2-YFP) and RNA polymerase (RNAP; β'-yGFP) in live Escherichia coli are measured by superresolution fluorescence microscopy. In moderate growth conditions, nucleoid-ribosome segregation is strong, and RNAP localizes to the nucleoid lobes. The mean copy numbers per cell are 4600 RNAPs and 55,000 ribosomes. Only 10-15% of the ribosomes lie within the densest part of the nucleoid lobes, and at most 4% of the RNAPs lie in the two ribosome-rich endcaps. The predominant observed diffusion coefficient of ribosomes is D(ribo) = 0.04 µm(2) s(-1), attributed to free mRNA being translated by one or more 70S ribosomes. We find no clear evidence of subdiffusion, as would arise from tethering of ribosomes to the DNA. The degree of DNA-ribosome segregation strongly suggests that in E. coli most translation occurs on free mRNA transcripts that have diffused into the ribosome-rich regions. Both RNAP and ribosome radial distributions extend to the cytoplasmic membrane, consistent with the transertion hypothesis. However, few if any RNAP copies lie near the membrane of the endcaps. This suggests that if transertion occurs, it exerts a direct radially expanding force on the nucleoid, but not a direct axially expanding force.
© 2012 Blackwell Publishing Ltd.
Figures



Comment in
-
Extending the tools of single-molecule fluorescence imaging to problems in microbiology.Mol Microbiol. 2012 Jul;85(1):1-4. doi: 10.1111/j.1365-2958.2012.08089.x. Epub 2012 May 17. Mol Microbiol. 2012. PMID: 22571513
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

