Colloidal aggregation affects the efficacy of anticancer drugs in cell culture
- PMID: 22625864
- PMCID: PMC3423826
- DOI: 10.1021/cb300189b
Colloidal aggregation affects the efficacy of anticancer drugs in cell culture
Abstract
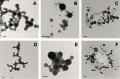
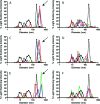
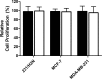
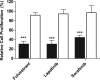
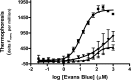
Many small molecules, including bioactive molecules and approved drugs, spontaneously form colloidal aggregates in aqueous solution at micromolar concentrations. Though it is widely accepted that aggregation leads to artifacts in screens for ligands of soluble proteins, the effects of colloid formation in cell-based assays have not been studied. Here, seven anticancer drugs and one diagnostic reagent were found to form colloids in both biochemical buffer and in cell culture media. In cell-based assays, the antiproliferative activities of three of the drugs were substantially reduced when in colloidal form as compared to monomeric form; a new formulation method ensured the presence of drug colloids versus drug monomers in solution. We also found that Evans Blue, a dye classically used to measure vascular permeability and to demonstrate the "enhanced permeability and retention (EPR) effect" in solid tumors, forms colloids that adsorb albumin, as opposed to older literature that suggested the reverse.
Figures

References
-
- Roche O.; Schneider P.; Zuegge J.; Guba W.; Kansy M.; Alanine A.; Bleicher K.; Danel F.; Gutknecht E. M.; Rogers-Evans M.; Neidhart W.; Stalder H.; Dillon M.; Sjogren E.; Fotouhi N.; Gillespie P.; Goodnow R.; Harris W.; Jones P.; Taniguchi M.; Tsujii S.; von der Saal W.; Zimmermann G.; Schneider G. (2002) Development of a virtual screening method for identification of “frequent hitters” in compound libraries. J. Med. Chem. 45, 137–142. - PubMed
-
- Feng B. Y.; Simeonov A.; Jadhav A.; Babaoglu K.; Inglese J.; Shoichet B. K.; Austin C. P. (2007) A high-throughput screen for aggregation-based inhibition in a large compound library. J. Med. Chem. 50, 2385–2390. - PubMed
-
- McGovern S. L.; Helfand B. T.; Feng B.; Shoichet B. K. (2003) A specific mechanism of nonspecific inhibition. J. Med. Chem. 46, 4265–4272. - PubMed
-
- McGovern S. L.; Caselli E.; Grigorieff N.; Shoichet B. K. (2002) A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J. Med. Chem. 45, 1712–1722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

