Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei
- PMID: 22626265
- PMCID: PMC3411448
- DOI: 10.1186/1742-2094-9-97
Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei
Abstract
Background: Chronic alcohol use changes the brain's inflammatory state. However, there is little work examining the progression of the cytokine response during alcohol withdrawal, a period of profound autonomic and emotional upset. This study examines the inflammatory response in the central nucleus of the amygdala (CeA) and dorsal vagal complex (DVC), brain regions neuroanatomically associated with affective and cardiorespiratory regulation in an in vivo rat model of withdrawal following a single chronic exposure.
Methods: For qRT-PCR studies, we measured the expression of TNF-α, NOS-2, Ccl2 (MCP-1), MHC II invariant chain CD74, and the TNF receptor Tnfrsf1a in CeA and DVC samples from adult male rats exposed to a liquid alcohol diet for thirty-five days and in similarly treated animals at four hours and forty-eight hours following alcohol withdrawal. ANOVA was used to identify statistically significant treatment effects. Immunohistochemistry (IHC) and confocal microscopy were performed in a second set of animals during chronic alcohol exposure and subsequent 48-hour withdrawal.
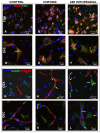
Results: Following a chronic alcohol exposure, withdrawal resulted in a statistically significant increase in the expression of mRNAs specific for innate immune markers Ccl2, TNF-α, NOS-2, Tnfrsf1a, and CD74. This response was present in both the CeA and DVC and most prominent at 48 hours. Confocal IHC of samples taken 48 hours into withdrawal demonstrate the presence of TNF-α staining surrounding cells expressing the neural marker NeuN and endothelial cells colabeled with ICAM-1 (CD54) and RECA-1, markers associated with an inflammatory response. Again, findings were consistent in both brain regions.
Conclusions: This study demonstrates the rapid induction of Ccl2, TNF-α, NOS-2, Tnfrsf1a and CD74 expression during alcohol withdrawal in both the CeA and DVC. IHC dual labeling showed an increase in TNF-α surrounding neurons and ICAM-1 on vascular endothelial cells 48 hours into withdrawal, confirming the inflammatory response at the protein level. These findings suggest that an abrupt cessation of alcohol intake leads to an acute central nervous system (CNS) inflammatory response in these regions that regulate autonomic and emotional state.
Figures


Similar articles
-
Rapid temporal changes in the expression of a set of neuromodulatory genes during alcohol withdrawal in the dorsal vagal complex: molecular evidence of homeostatic disturbance.Alcohol Clin Exp Res. 2012 Oct;36(10):1688-700. doi: 10.1111/j.1530-0277.2012.01791.x. Epub 2012 Apr 6. Alcohol Clin Exp Res. 2012. PMID: 22486438 Free PMC article.
-
Coordinated dynamic gene expression changes in the central nucleus of the amygdala during alcohol withdrawal.Alcohol Clin Exp Res. 2013 Jan;37 Suppl 1(0 1):E88-100. doi: 10.1111/j.1530-0277.2012.01910.x. Epub 2012 Jul 24. Alcohol Clin Exp Res. 2013. PMID: 22827539 Free PMC article.
-
Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei.Brain Behav Immun. 2004 Mar;18(2):123-34. doi: 10.1016/j.bbi.2003.09.004. Brain Behav Immun. 2004. PMID: 14759590
-
NIH conference. Alcohol withdrawal and noradrenergic function.Ann Intern Med. 1987 Dec;107(6):875-89. doi: 10.7326/0003-4819-107-6-875. Ann Intern Med. 1987. PMID: 2825572 Review.
-
Involvement of the Dorsal Vagal Complex in Alcohol-Related Behaviors.Front Behav Neurosci. 2022 Mar 7;16:801825. doi: 10.3389/fnbeh.2022.801825. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35330845 Free PMC article. Review.
Cited by
-
Chronic intermittent ethanol induced axon and myelin degeneration is attenuated by calpain inhibition.Brain Res. 2015 Oct 5;1622:7-21. doi: 10.1016/j.brainres.2015.06.014. Epub 2015 Jun 20. Brain Res. 2015. PMID: 26100335 Free PMC article.
-
The effect of neuroimmune modulation on subjective response to alcohol in the natural environment.Alcohol Clin Exp Res. 2022 May;46(5):876-890. doi: 10.1111/acer.14821. Epub 2022 Apr 11. Alcohol Clin Exp Res. 2022. PMID: 35362101 Free PMC article. Clinical Trial.
-
A Pivotal Role for Thiamine Deficiency in the Expression of Neuroinflammation Markers in Models of Alcohol-Related Brain Damage.Alcohol Clin Exp Res. 2019 Mar;43(3):425-438. doi: 10.1111/acer.13946. Epub 2019 Jan 20. Alcohol Clin Exp Res. 2019. PMID: 30589435 Free PMC article.
-
Investigation and analysis of factors related to sleep conditions during the acute withdrawal period of alcohol use disorder.Front Psychiatry. 2025 May 6;16:1469324. doi: 10.3389/fpsyt.2025.1469324. eCollection 2025. Front Psychiatry. 2025. PMID: 40395471 Free PMC article.
-
Aging with alcohol-related brain damage: Critical brain circuits associated with cognitive dysfunction.Int Rev Neurobiol. 2019;148:101-168. doi: 10.1016/bs.irn.2019.09.002. Epub 2019 Oct 17. Int Rev Neurobiol. 2019. PMID: 31733663 Free PMC article. Review.
References
-
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;7:4733–4744. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

