Emerging concepts in haematopoietic cell transplantation
- PMID: 22627859
- PMCID: PMC4006975
- DOI: 10.1038/nri3226
Emerging concepts in haematopoietic cell transplantation
Abstract
Haematopoietic cell transplantation (HCT) is the most widely used form of cellular therapy. It is the only known cure for some haematological malignancies and has recently been used in additional clinical settings, such as allograft tolerance induction and treatment of autoimmune diseases. Recent advances have enabled HCT in a wider range of patients with improved outcomes. This Review summarizes the latest developments in this therapy, focusing on issues that will affect future advancement.
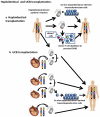
Figures



References
-
- Barker JN, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

