The impact of infection and tissue damage in solid-organ transplantation
- PMID: 22627862
- PMCID: PMC4053185
- DOI: 10.1038/nri3215
The impact of infection and tissue damage in solid-organ transplantation
Abstract
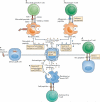
Investigations over the past two decades are revealing complexities in the regulation of the innate immune response, and how this response, in turn, controls adaptive immunity. Microbial exposure, infections and tissue damage that accompany solid-organ transplantation result in the release of pathogen- and damage-associated molecular patterns, as well as pathogen- or allograft-derived antigens. Here, we review these triggers of innate and adaptive immunity, and discuss emerging paradigms of the many ways in which infections and tissue damage might directly or indirectly affect alloreactivity and the outcome of transplanted allografts.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

